Oxygen is an essential element for various scientific and industrial processes. In the laboratory, it is commonly prepared using a few established methods, each leveraging chemical reactions to produce this vital gas.
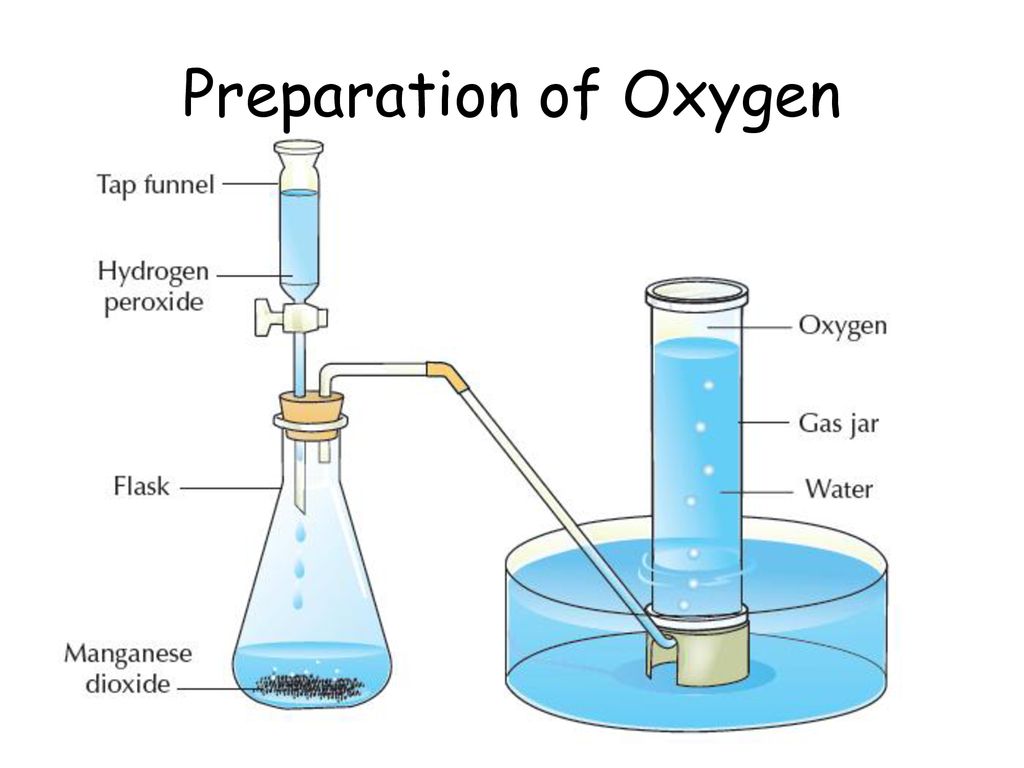
1. From Hydrogen Peroxide
The most common method of preparing oxygen in the lab is catalytic decomposition of hydrogen peroxide.
At room temperature, hydrogen peroxide decomposes slowly to form oxygen and water as shown below:
Hydrogen Peroxide → Water + Oxygen
2H20 (aq) → 2H20 (l) +O2 (g)
However, the rate of decomposition under such conditions is too slow that no meaningful amount of oxygen can be produced.
To speed up the decomposition process (and release more oxygen), manganese (IV) oxide is often added as a catalyst. This means that manganese (IV) oxide doesn’t take part in the reaction but only speeds it up.
The produced oxygen is collected by downward displacement of water (or displacing water from an inverted jar) because it is slightly soluble in water.
2. From Sodium Peroxide
The experimental setup of this method is similar to the one for hydrogen peroxide above. However, a small amount of powdered sodium peroxide is put in the flask and distilled water is dropped through the thistle funnel.
Sodium peroxide reacts with water forming oxygen, which is then collected by downward displacement of water.
Sodium Peroxide + Water → Sodium hydroxide + Oxygen
2Na2O2 (s) + 2H2O (l) → 4NaOH (aq) + O2 (g)
3. Heating of Potassium Chlorate, Metal Nitrates, Or Potassium manganete (VII)
I. Potassium Chlorate
Potassium Chlorate → Potassium Chloride +Oxygen
II. Decomposition of metal nitrates
Sodium nitrate → Sodium nitrite + Oxygen
III. Heating Potassium manganete (VII)
Potassium manganete (VII) → Potassium manganete (VI) + manganese (IV) oxide +Oxygen
6. Electrolysis of water
Water (H₂O) is decomposed into oxygen and hydrogen gases by passing an electric current through it. The chemical equation for this reaction is:
2H2O→2H2+O2
In practice, water is often mixed with a small amount of sulfuric acid to increase its conductivity. Electrodes are placed in the water, and an electric current is applied, causing water to split into hydrogen and oxygen. The oxygen gas is collected at the positive electrode (anode).
Dying of Oxygen
Oxygen prepared by the first two methods is often moist since they are bubbled through water (i.e downward displacement of water).
If it is necessary to obtain dry oxygen, then produced oxygen is further bubbled through wash bottle containing a drying agent like concentrated sulphuric acid, which dries the gas by absorbing moisture present.
Alternatively, the gas is passed through anhydrous calcium chloride or phosphorous pentoxide, which are also great drying agents.
Image source: 1



