In the previous section, we defined a compound as a pure substance made up of two or more elements chemically combined. Given the many ways in which elements can combine, the number of possible compounds is virtually limitless.
But how do we name all these compounds? How can we know which elements constitute a compound?
Well, one simple way is to examine the suffixes attached to the names. These suffixes include “ate,” “ite,” and “ide”.
Here are a few basic rules regarding the use of these suffixes that you should keep in mind.
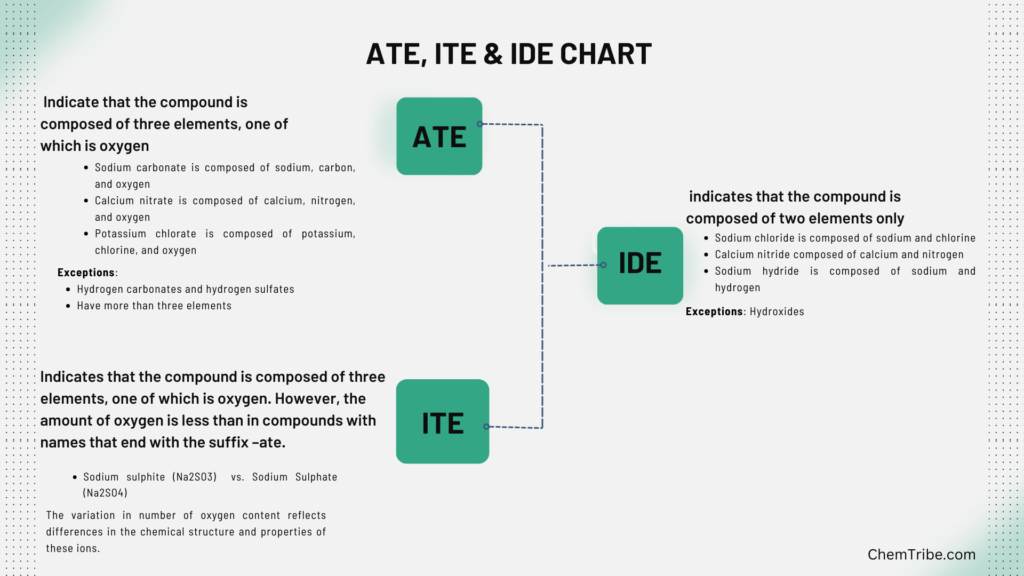
-ide
Compound names ending with the suffix –ide indicates that the compound is composed of two elements only. So:
- Sodium chloride is composed of sodium and chlorine
- Iron (II) sulphide is composed of iron and Sulphur
- Calcium nitride composed of calcium and nitrogen
- Sodium hydride is composed of sodium and hydrogen
- Calcium carbide is composed of calcium and carbon.
An exception to this rule is hydroxides. Hydroxides (such as sodium hydroxide (NaOH) or calcium hydroxide (Ca(OH)₂), are composed of three elements—hydrogen, oxygen, and a metal.
But the suffix “-ide” is still used in their names because the hydroxide ion (OH⁻) behaves as a single entity in chemical reactions, making it convenient to treat it as a single unit despite its composite structure.
-ate
Compound names ending with suffix –ate indicate that the compound is composed of three elements, one of which is oxygen. So:
- Sodium sulphate is composed of sodium, Sulphur, and oxygen
- Sodium carbonate is composed of sodium, carbon, and oxygen
- Calcium nitrate is composed of calcium, nitrogen, and oxygen
- Potassium chlorate is composed of potassium, chlorine, and oxygen
Exceptions to this rule are hydrogen carbonates and hydrogen sulphates. These have more than three elements. For instance, sodium bicarbonate (NaHCO₃) and sodium bisulfate (NaHSO₄), contain hydrogen, sodium, carbon (in the case of NaHCO₃)), sulfur (in the case of NaHSO₄), and oxygen.
The exceptions occur because hydrogen carbonates and hydrogen sulfates are derived from the corresponding carbonates and sulfates by replacing one of the oxygen atoms with a hydrogen atom.
Despite containing more elements than the typical “-ate” compounds, they retain the “-ate” suffix to indicate their relationship to the parent compounds and their oxygen-containing nature.
-ite
Compound names ending with –ite indicates that the compound is composed of three elements, one of which is oxygen. However, the amount of oxygen is less than in compounds with names that end with the suffix –ate.
- Sodium sulphite (Na2SO3) vs. Sodium Sulphate (Na2SO4)
- Calcium sulphite (CaSO3·x(H2O)) vs. Calcium sulphate (CaSO4 )
This variation in number of oxygen content reflects differences in the chemical structure and properties of these ions.
Summary