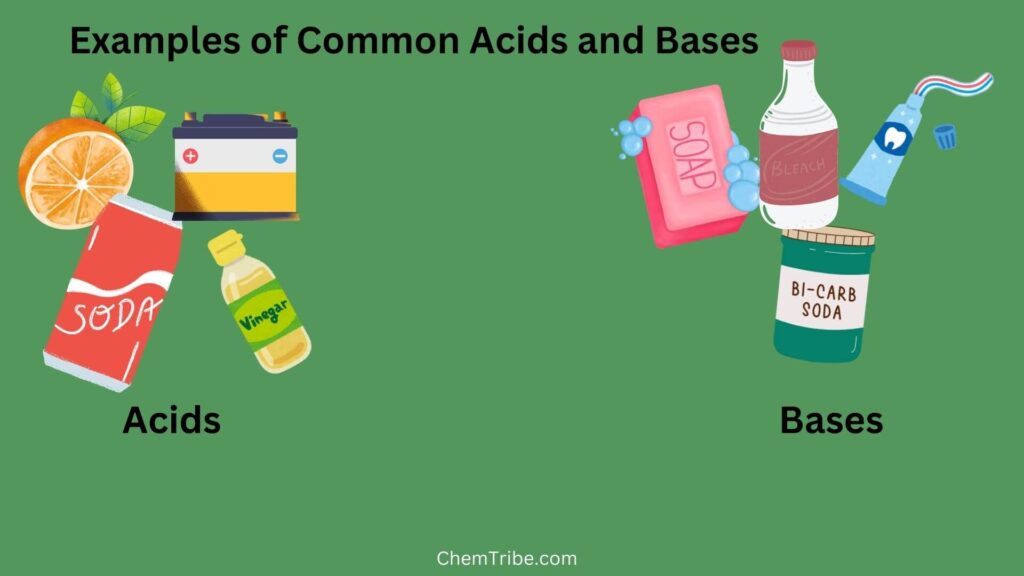
In the previous post, we mentioned that one of the properties of acids is that they have sour tastes, while bases have bitter tastes. Using taste to classify substances as acids or bases is not an accurate method. It is not safe either due to the potential harm that acidic or basic substances can cause to the mouth and digestive system. Taste perception can also vary among individuals.
The most reliable method for determining whether a substance is acidic or basic is by using acid-base indicators or pH indicators.
To indicate means to be a sign of something. So, acid-base indicators signal that something is an acid or base. So, we can say that pH indicators are substances that give certain colors when mixed with acids and other colors when mixed with bases.
Types of Indicators
There are several types of indicators. The most common types that you are likely to come across in the laboratory are
- Natural pH indicators
- Commercial indicators
- Universal indicators.
Natural pH Indicators
Essentially, all the above-mentioned types of indicators are colored dyes, which are obtained mainly from plants.
A readily available source of dyes that we can use as indicators are flowers and leaves of certain plants. These include hibiscus flowers, tradescantia, beetroot, blueberry, turmeric, and red cabbage leaves among others. You can easily extract dyes from these flowers and leaves via solvent extraction.
These extracts give distinct colors in acids and bases as shown below. We can therefore use them as indicators.
| Plant Extract | Color in Acid | Color in base |
| Red Cabbage | Red | Blue |
| Hibiscus | Red | Yellow |
| Tumeric | Yellow | Red-Ornage |
| Beetroot | Red | Purple |
| Rose Petals | Red | Blue |
| Petunia Petals | reddish-purple | violet |
| Blueberry | Red | Blue |
The composition of flower extracts continuously changes with time, making the color of the extract to change as well. This implies that the mixture of the extract and acid or base also changes color with time. Because of this instability, natural pH indicators (aka flower and leave extracts) give inconsistent results when used as acid-base indicators. For the best results, flower extracts should be used as indicators when they are freshly prepared.
Apart from stability, it is also difficult to standardize plant extracts. This makes it difficult to compare results across different studies or laboratories.
Commercial Indicators
In most laboratory settings, commercial indicators, which give more consistent results are often used. They are more stable and last for a long without changing color.
The litmus indicator is one of the most commonly used commercial acid-base indicators. It turns red in acids and blue in bases. Litmus can be used as either a solution or paper (commonly referred to as litmus paper).
Other commercial acid-base indicators include phenolphthalein, methyl orange, and bromothymol blue. Here are their respective colors in acids and bases.
| Indicator | Color in acid | Color in base |
| Litmus | Red | Blue |
| Phenolphthalein | Colourless | Pink |
| Bromothymol blue | Yellow | Blue |
| Methyl orange | Red | Yellow |
Universal Indicators
The two types of indicators that we have discussed so far provide no information about the strength of an acid or base. However, as we discussed in the previous post, some acids and bases are stronger while others are weak.
To determine the strength of an acid or a base, a universal indicator is used. It comprises of a mixture of indicators. It exhibits a range of colors in acids and bases depending on the strength of the solution (as shown below). This allows for more precise and detailed pH measurements compared to natural and commercial indicators.
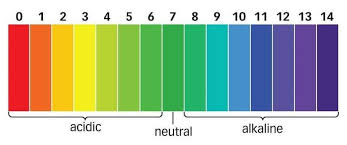
So, with a universal indicator (like the one shown above), you can quickly and easily determine the approximate pH of a solution by observing the color change, ranging from red (indicating strong acidity) to lime green (indicating slightly acidic), green(indicating neutral), blue (indicating slightly alkaline) to dark blue (indicating strong alkalinity). This versatility makes universal indicators suitable for a wide range of applications in laboratories, educational settings, and industrial processes where accurate pH measurements are required.
Universal Indicator is available in two forms: as a liquid or strips of paper. It is usually sold with a colour chart, which showcases a range of colors depending on the corresponding strength of an acid or base (pH range/scale).
pH scale
pH scale is used to measure the strength of acids (acidity) and strength of bases (alkalinity). It has values that range from 0 to 14.
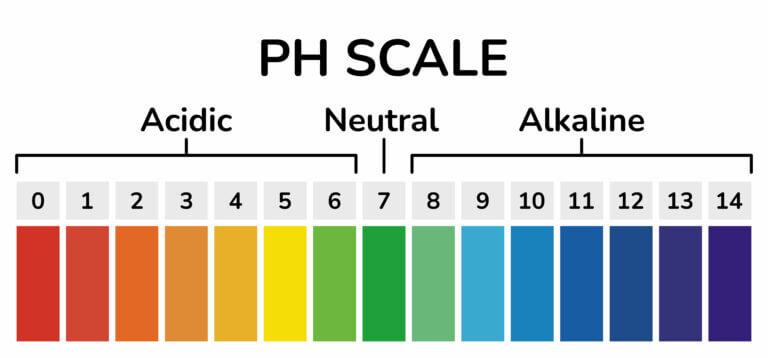
The pH values of acid range from 0 to values just less than 7. The lower the pH value, the stronger or more acidic the solution is. Solutions of sulphuric acid, hydrochloric acid, and nitric acids have pH values of about 1 and are therefore considered to be strong acids. Lemon juice and ethanoic acid have pH values that range between 4 and 7, and are considered to be weak acids.
Substances with a pH value of 7 are neither acidic nor basic. They are neutral. Examples include distilled water and sodium chloride.
pH value of bases ranges between 7 and 14. The higher the pH value, the more alkaline the solution is. Solutions of ammonia, calcium hydroxide, and sodium hydrogen carbonate have pH values that range between 7 and 10 and are weak bases. Solutions of sodium hydroxide and potassium hydroxide have pH values ranging from 10 to 14 and are strong bases.