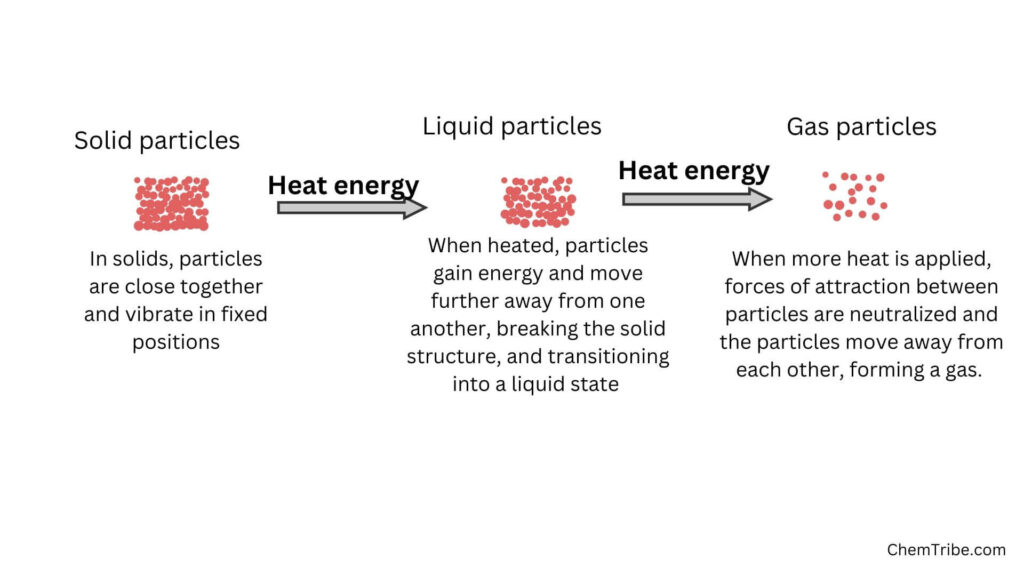
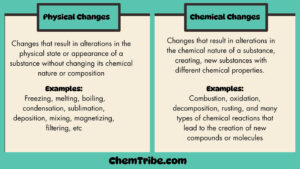
Matter exists in three main states depending on environmental conditions like temperature or pressure. These include Solid, Liquid, and Gaseous states. These three states of matter have different properties.
But how well do you understand the differences between the different properties of these states of matter? Can you accurately tell whether a property belongs to a solid, liquid, or gaseous state? Do you know other states of matter besides these three?
Let’s test your knowledge! You can also challenge others on this states of matter quiz to see who knows more.
Have fun while learning this interesting chemistry concept. Do not forget to share.
Should you encounter any confusion with a particular question or answer, don’t hesitate to brush up on your knowledge by revisiting these posts: