Generally, ionization energy increases across a period from left to right. If you need a quick refresher, here’s why:
As you move across a period, the nuclear charge increases, but the number of energy levels stays the same. At the same time, the atomic size decreases, pulling the electrons closer to the nucleus. Because the electrons are held more tightly, removing one becomes harder—so the ionization energy rises.
Anomalies
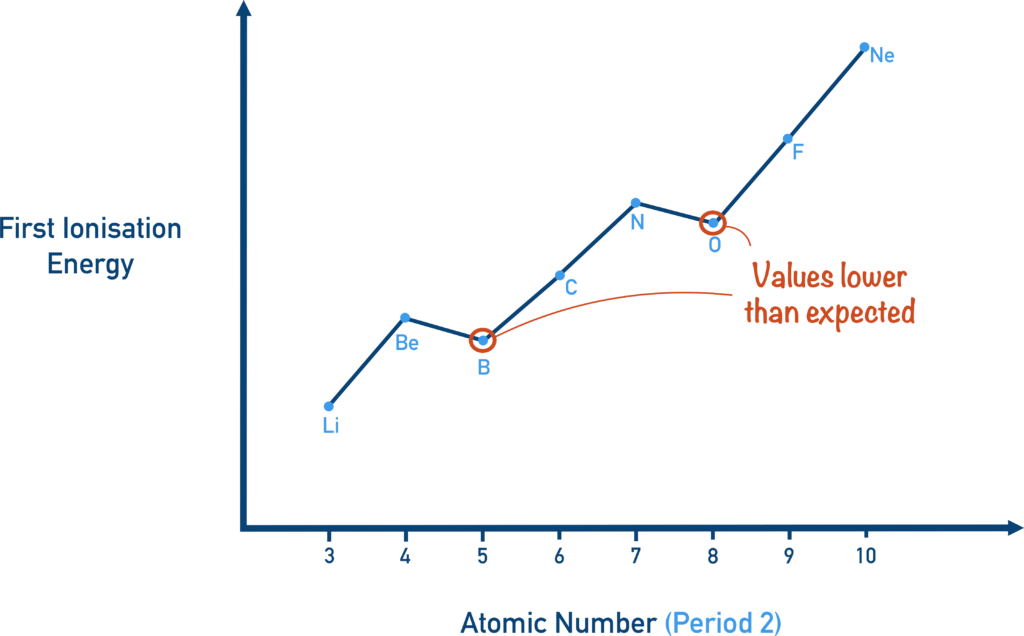
However, there are a few anomalies as you move across a period from left to right. Take Period 2, for example. As we go from Li to Be, the ionization energy increases, and this follows the expected trend. We can easily explain it by the higher nuclear charge and the smaller atomic size, both of which cause the electrons to be held more tightly.
As we move from Be to B, there is a slight decrease in ionization energy, even though the nuclear charge increases and the atomic size becomes smaller. This anomaly can be explained by three key factors:
- Electronic configuration: Boron’s arrangement (1s² 2s² 2p¹) is less stable than beryllium’s fully filled 2s subshell (1s² 2s²). A less stable configuration makes it easier to remove an electron.
- Orbital type: Boron’s outermost electron occupies a 2p orbital, which is less penetrating and therefore less tightly held than the 2s orbital that holds Beryllium’s outermost electron.
- Shielding effect: Boron’s outer electron is shielded by four inner electrons, while Beryllium’s is shielded by only two. More shielding reduces the effective nuclear charge felt by the electron, making it easier to remove.
These factors together explain the unexpected dip in ionization energy from Be to B.
Because of these three factors, the outermost electron in Boron is not held as tightly by the nucleus and is therefore easier to remove. A similar dip in ionization energy appears in Period 3 when moving from Mg to Al, for the very same reasons.
As we move from B to C, the ionization energy increases again because the nuclear charge continues to rise and the atomic size decreases, making the electrons more tightly held.
However, when we go from N to O, the ionization energy dips once more. This happens because nitrogen has a more stable electronic arrangement:
N: 1s² 2s² 2p¹ 2p¹ 2p¹ (all three p orbitals are half-filled). A half-filled subshell is especially stable.
In oxygen, the configuration is: O: 1s² 2s² 2p² 2p¹ 2p¹. The p orbitals are neither half-filled nor fully filled, making this arrangement less stable. The paired electrons in one of the p orbitals also experience extra repulsion, making it easier to remove an electron.
A similar pattern appears in Period 3, where sulfur has a lower ionization energy than phosphorus for the same reason.
After this anomaly, the ionization energy increases as expected from O → F → Ne, following the rise in nuclear charge and decrease in atomic size.
Image source: 1



