In our definition of chemistry, we stated that:
- The substances that we encounter in our daily lives are in the form of what’s commonly referred to as matter.
- Anything you can see or touch around you—soil, rocks, air, water, plants, animals—is made of matter.
- So, matter is basically everything that makes up our universe.
Now there is another important aspect of matter that you need to know: all forms of matter have two properties in common: they occupy space and have mass.
Mass is the measure of the quantity of matter in an object. In other words, it is what gives an object its weight. When we weigh things, we are simply trying to figure out their mass. The more matter an object has, the more it will weigh.
To this end, we can deduce that:
Matter is anything that has mass and occupies space.
Primary States of Matter
Matter exists in three main states depending on environmental conditions like temperature or pressure. These include:
- Solid
- Liquid
- Gaseous
The three states of matter have different properties which we will discuss below.
Before then, we would like to bring to your attention that there are other states of matter apart from the above three. These are plasma and Bose-Einstein condensate. We have a post dedicated to these exotic states of matter. Feel free to check it out here.
Properties of Different States of Matter
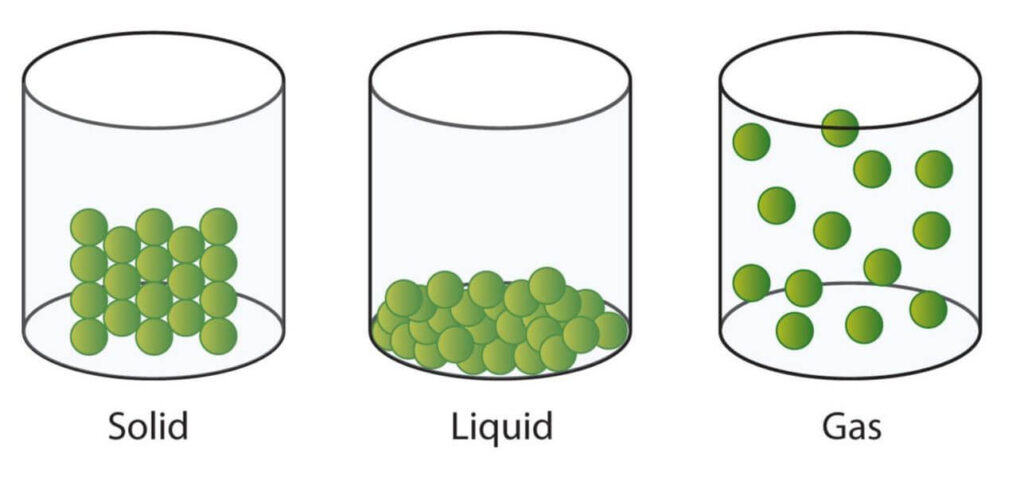
Solids
Solids have definite mass, volume, and shape
Solids are rigid and consist of particles tightly packed together. Consequently, they don’t move much but vibrate in place. It’s also difficult to compress their particles. These characteristics give solids definite mass and volume (volume being the amount of space occupied by a substance). Their limited movement also means they maintain their shape and don’t conform to the shape of the container they’re placed in. So, they also have definite shapes.
With that said, the shapes of solids can be altered by applying strong forces. For example, you can flatten a round piece of metal by hammering it. However, the volume will stay the same even after changing its shape through hammering.
Examples of solids include rocks, wood, solid ice, etc.
Liquids
Liquids have definite mass and volume but indefinite shapes
Particles in liquids are more loosely packed compared to solids, allowing them to flow around each other. Consequently, liquids will conform to the shape of the container they’re in, meaning they have indefinite shapes. However, compressing them is difficult because their particles remain close together, leaving little space between them to move. Therefore, like solids, liquids have fixed volumes.
Examples include water, milk, coffee, kerosene, etc.
Gases
Gases have indefinite shapes and volumes but definite mass
Gases have a significant amount of space between their particles. When not confined, gas particles tend to spread indefinitely, and when confined, they expand to fill the space or container they’re in. This is why gases have neither a definite shape nor volume. However, they do have a definite mass.
Examples include air, oxygen, carbon dioxide, nitrogen, etc.
State Transitions
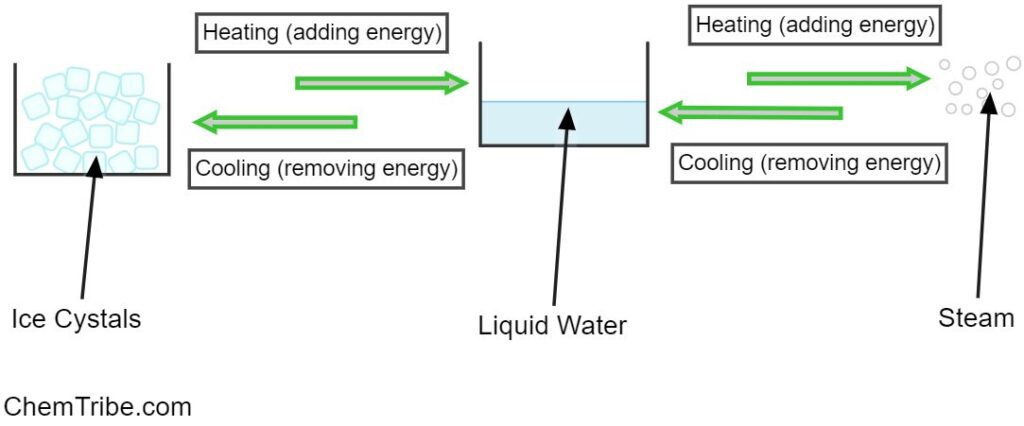
Most substances can exist in the three states mentioned above depending on environmental conditions such as temperature or pressure. For example, water can exist as a liquid at room temperature. If it is cooled, it freezes to form ice, which is water in its solid state. Conversely, if you heat water, it boils and forms vapor or steam, which is water in its gaseous state.
There are also many other substances that exist as solids at room temperature but can be converted into liquids by heating them. For example, a piece of iron can be heated to very high temperatures until it melts into a liquid. Most of the metals that we encounter in our day-to-day lives can also be transformed into liquids if they are heated sufficiently.
Similarly, substances that exist as gases at room temperature can be converted to liquids by cooling. For example, if air is cooled to very low temperatures, it forms liquid air. Gases can also be transformed into liquids by compressing them (or increasing their pressure).
In summary, adding or removing energy from matter causes it to transition from one state to another. For example, we have observed that heating water (adding thermal energy) converts it into a gaseous state, while cooling it (removing energy) converts it into ice (solid state). State transitions can also be induced by changing pressure and motion, particularly in gases.