Crystallization is another separation technique that chemists use to separate solids or soluble substances from their solutions. Generally, crystallization is a process that occurs when a substance solidifies from a liquid or precipitates out of a liquid or a gas. It is this process that chemists leverage in crystallization as a separation technique.
Many solids exist naturally in the form of regularly shaped structures called crystals. Such solids are said to be crystalline and most of them are soluble in water. Examples include common salt, sugar, quartz, and diamond among others.
And speaking of solubility in water, there is a limit to the amount of solid that can dissolve in a given volume of water. A solution in which no more solute can dissolve is said to be saturated while a solution that can take more solute is said to be unsaturated.
For many crystalline solids, the mass that dissolves in a given volume of water to give a saturated solution increases with increase in temperature. For instance, the mass of potassium nitrate that dissolves in a given volume of water increases as temperature is increased as shown in the table below:
| Temperature (0C) | Mass Dissolved (g) |
| 20 | 33 |
| 30 | 47 |
| 40 | 65 |
| 50 | 84 |
| 60 | 107 |
Crystallization as a separation technique leverages this increase in solubility with temperature: if a saturated solution at a higher temperature is allowed to cool, the solute will no longer be soluble in the solvent and will form crystals.
In the table above, for instance, a hot saturated solution of potassium nitrate (at 600C) contains more solute than a colder saturated solution (at 200C). When a hot saturated solution of potassium nitrate is cooled, the amount of solute that it can hold decreases. The excess solute comes out of the solution and appears as crystals.
Cooling Crystallization
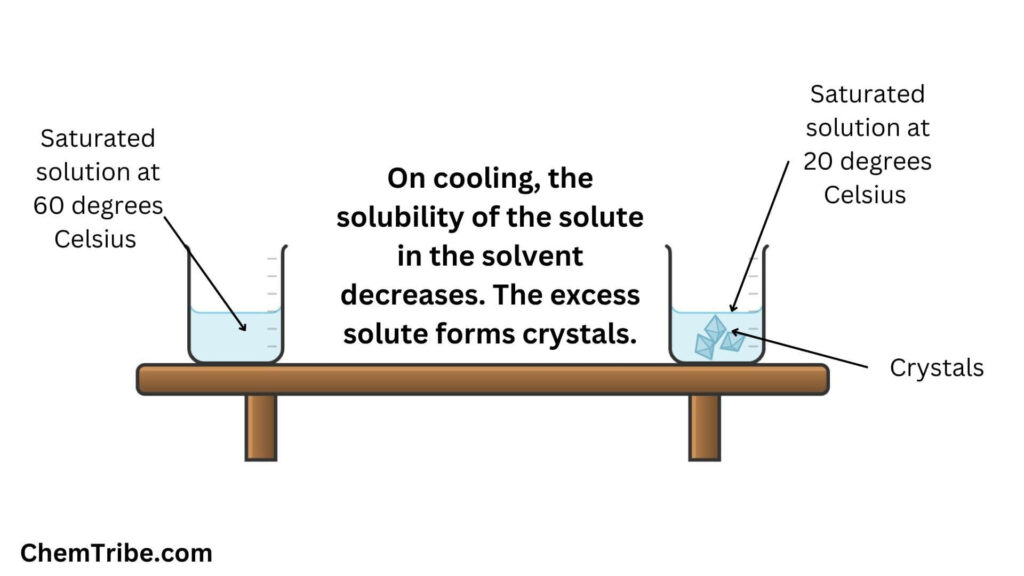
The above method of crystallization where crystallization is induced through cooling is commonly referred to as Cooling Crystallization. This is how it works in a nutshell: a hot saturated solution containing the dissolved substance is allowed to cool gradually. As the temperature decreases, the solubility of the solute decreases as well, leading to the formation of crystals. These crystals precipitate out of the solution and can be collected by filtration or centrifugation.
Evaporative Crystallization
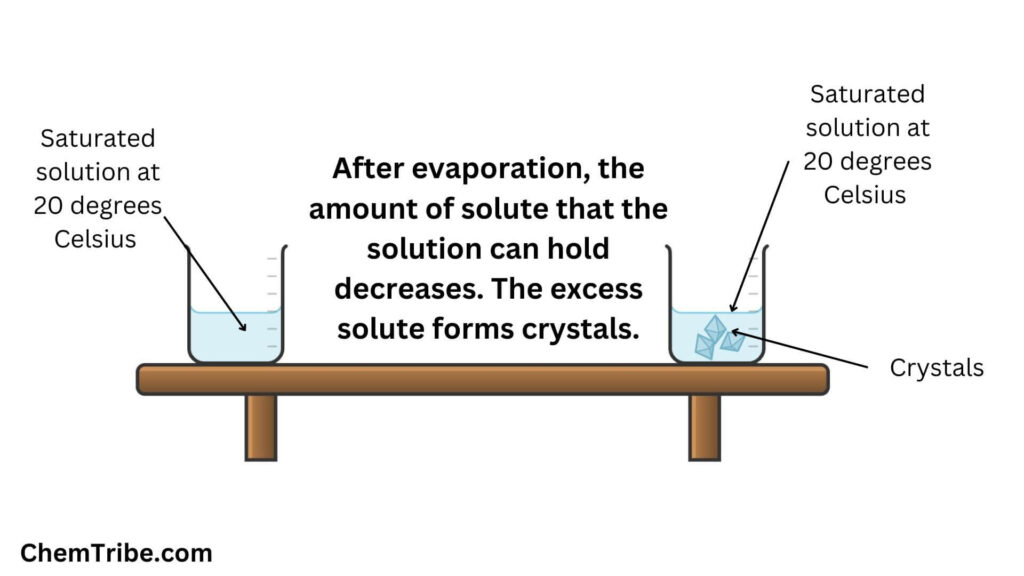
Crystals can also be formed by evaporating the saturated solution. As some of the water in the saturated solution evaporates, the amount of solute that the solution can hold decreases. The excess solute comes out of the solution and appears in the form of crystals.
This type of crystallization which is induced through evaporation is commonly referred to as Evaporative Crystallization. This is how it works in a nutshell: A solution containing the dissolved substance is heated or subjected to reduced pressure to increase the rate of evaporation. As the solvent evaporates, the concentration of the solute in the solution increases until it reaches the point of saturation. At this point, the solute begins to crystallize out of the solution, forming solid crystals. These crystals can be separated from the remaining solution through filtration or centrifugation.
Size of Crystals
The size of crystals formed in either crystallization method depends on the rate of cooling (in cooling crystallization) or the rate of evaporation (in evaporative crystallization).
If cooling occurs rapidly, many small crystals are formed. And if it occurs slowly, very few but large crystals are formed.
Conversely, if evaporation occurs rapidly, very few crystals are formed. In fact, if you do it by boiling off the solution, you will end up with very small crystals that appear in the form of a powder. If the evaporation is allowed to take place slowly at room temperature, a few but large crystals are formed.
Common Application of Crystallization as a Separation Technique
- Extraction of salt from salty water.
- Extraction of sugar from sugar cane juice.
- Preparation of crystals of alum from a solution containing impure alum
- Extraction of medicinal substances (active ingredients) from plants.
- Isolation of natural products, such as vitamins, essential oils, and plant extracts, from complex mixtures.
- Production of high-purity chemicals and intermediates in industries such as specialty chemicals, flavors, fragrances, and dyes.
Further Reading:
How to Separate Copper Sulphate from Aqueous Copper Sulphate



