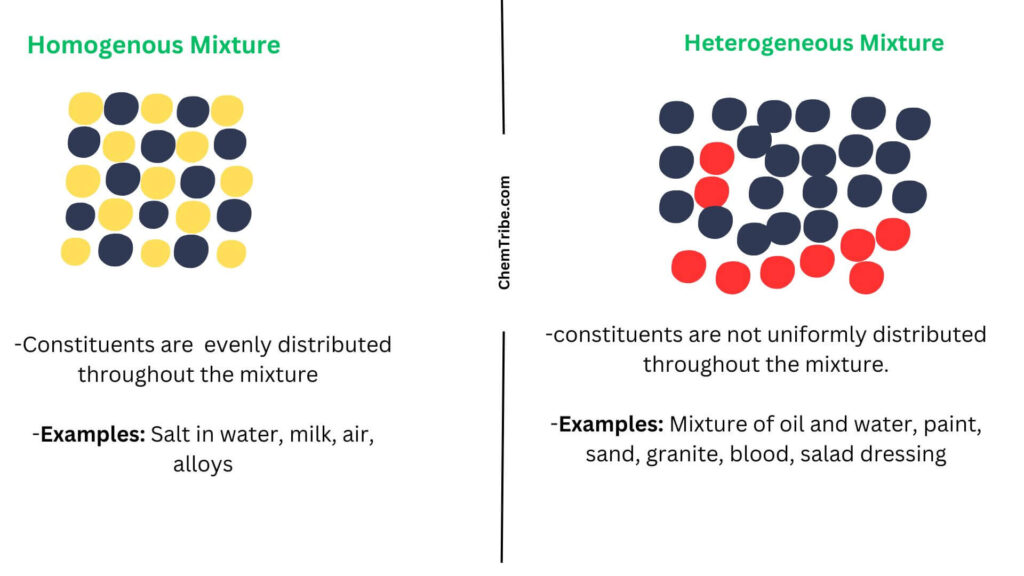
A solvent is a substance, typically a liquid, which can dissolve other substances, called solutes, to form a homogeneous mixture known as a solution. Solvents are capable of breaking down the chemical bonds between solute particles, allowing them to disperse and become evenly distributed throughout the solvent.

When you add ethanol to water, they mix completely to form a uniform mixture. Essentially, ethanol dissolves in water to form a solution. In this case, water is a solvent.
While water is the most common liquid solvent, there are many other liquid solvents that you will come across in today’s chemical labs, including methanol, ethanol, acetone, benzene, toluene, chloroform, diethyl ether dichloromethane, tetrachloroethylene, etc, all of which dissolve a variety of other substances.
The above examples are liquid solvents. Solvents can also be gases and even solids.
Gases always mix completely forming a solution. For instance, air is a solution of several gases in nitrogen. In this case, nitrogen, which is the major component of air can be described as the solvent and the other gases are solutes.
Alloys, which are uniform mixtures of metals can also be considered to be solutions. For instance, bronze is a solution of copper and tin and brass is a solution of zinc and copper.
In either case, one metal is a solvent (the one in larger volume or proportion) and it allows the other metal (solutes) to disperse uniformly in it, resulting in a homogeneous solid mixture (alloy) with unique properties.