Prerequisite Reading: Distillation (as separation technique)
After learning about distillation in the previous section, you might be wondering about its practical applications. Well, distillation plays a critical role in many industrial processes, particularly those involving the purification of substances, concentration of solutions, and extraction of valuable components from complex mixtures. Here are some of the areas where distillation is applied in real life:
Water Purification
Simple distillation is used in the production of distilled water, which we use in our laboratories and in the manufacturing of bottled pure water. By boiling water to produce steam, impurities are left behind, and the steam is condensed back into liquid form, resulting in distilled water that is free from contaminants such as minerals, bacteria, and pollutants.
Alcohol Production
Distillation is applied in the production of alcoholic beverages, especially spirits (whiskey, brandy, rum, gin, etc). Powdered starchy food materials are mashed with water and allowed to ferment. This converts some of the sugars to ethanol. The mixture is filtered to obtain a filtrate, which is a dilute solution of ethanol and water. The solution is distilled to obtain spirits, which are concentrated solutions of ethanol in water.
Petroleum Refining
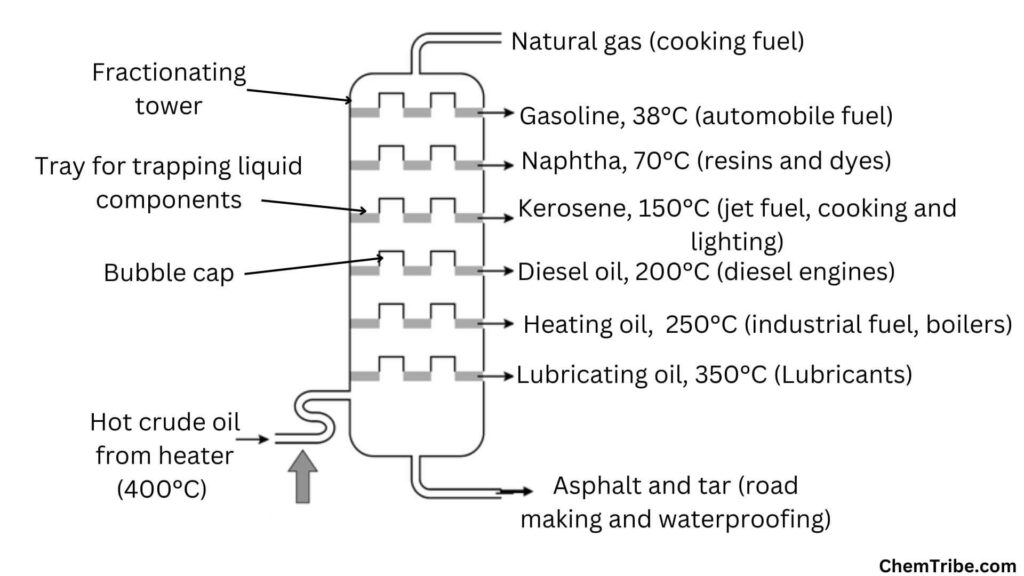
In the petroleum industry, crude oil undergoes fractional distillation to separate it into various fractions with different boiling points, such as gasoline, diesel, and kerosene. For this, a fractional distillation column, also referred to as a fractionating tower is used. Hot crude oil, with a temperature of approximately 400°C, is introduced at the bottom of the tower. Components with the highest boiling points, such as asphalt and tar, remain as liquids and are collected at the bottom. Other components vaporize and ascend the tower. As they rise, the vapors pass through a series of bubble caps and trays, cooling in the process. Components with higher boiling points, like lubricating oil, fuel oil, and diesel oil, condense into liquids in the middle of the tower and are collected on trays. Those with lower boiling points, such as kerosene and gasoline, condense near the top and are also collected. Finally, natural gas, with the lowest boiling point, is removed as gas at the top of the tower.
Production of Oxygen and Nitrogen from Atmospheric Air
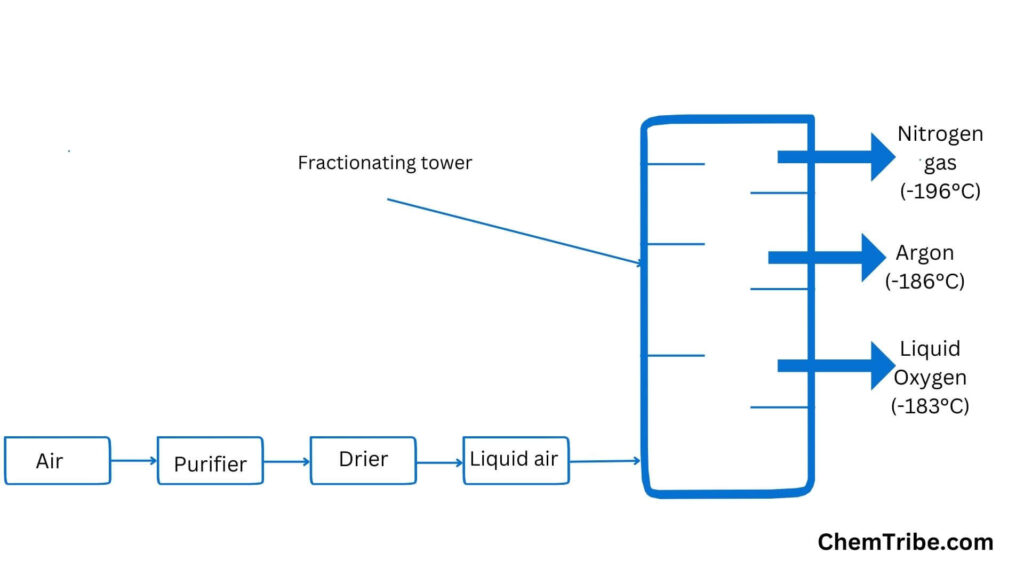
Distillation is also used in the production of oxygen and nitrogen from atmospheric air. Carbon dioxide (CO2) is initially removed by passing air through a solution of sodium hydroxide. Next, water vapor is eliminated by either passing the air through a drying agent like sulfuric acid or cooling it to -25°C to freeze water as ice. The remaining air undergoes a continuous process of expansion, cooling, and compression to liquefy it (liquefaction). Subsequently, further cooling is carried out to a temperature of -200°C. Upon warming, the following natural gases are separated at the specified temperatures:
- Nitrogen at -196°C
- Argon at -186°C
- Oxygen at -183°C
Food Processing
Distillation is used in food processing industries to concentrate flavors and aromas, as well as to separate volatile compounds from food products. In concentrating fruit juices, for instance, the juice is heated in a vacuum still, reducing its volume while preserving its flavor and nutritional properties. The concentrated juice is then reconstituted with water before packaging or used as a flavoring ingredient in various food products. Distillation is also applied in removing water from dairy products and soups, to extend their shelf life and enhance their flavor. By heating the food under reduced pressure, water evaporates, leaving behind concentrated products with improved stability and flavor.
Pharmaceutical Manufacturing
Distillation is extensively used in the pharmaceutical industry for various purposes, ranging from the purification of chemicals to the production of essential pharmaceutical products. For instance, solvent-based distillation processes are used for blending, granulation, coating, and drying of pharmaceutical ingredients to produce finished dosage forms with precise composition and quality. Distillation is also used to purify solvents and reagents used in pharmaceutical manufacturing processes. After various pharmaceutical reactions or extractions, solvents are distilled to separate them from reaction products or extractants. The recovered solvents can be reused, reducing waste generation and production costs.
Recycling of Oils
Distillation is also used in recycling oils by removing contaminants such as water, dirt, and impurities, thereby prolonging the oil’s viability for reuse. Through heating, contaminants vaporize and separate from the oil. Fractional distillation further refines the process by separating contaminants based on their differences in boiling points. After condensation, purified oil is collected separately, while contaminants are disposed of.