In chemistry, elements are represented by letters. These letters are called chemical symbols. As of February 2024, 118 chemical elements had been identified and named officially by the International Union of Pure and Applied Chemistry (IUPAC). Each of these elements is represented by a chemical symbol.
Chemical symbols consist of one or two letters, which are usually the first letter or the first and another letter of the element’s English or Latin name. Another letter here means that the second letter can be derived from any letter of the English or Latin name and not necessarily the second letter of the name.
The first letter of a chemical symbol is always capitalized, while the second letter is always lowercase. This is done to avoid confusion with other notations in chemistry. For instance, “Co” represents the element cobalt, whereas “CO” stands for the compound carbon monoxide, composed of carbon (C) and oxygen (O) atoms.
Here are quick examples of elements whose symbols are derived from the first letters of their English names.
| Element | Symbol |
| Carbon | C |
| Nitrogen | N |
| Oxygen | O |
| Hydrogen | H |
| Fluorine | F |
| Iodine | I |
| Phosphorus | P |
In cases where multiple elements start with the same letter, chemists found it necessary to represent some elements with two letters, as shown in the table below. Remember that the second letter in a chemical symbol is always lowercase.
| Element | Symbol |
| Calcium | Ca |
| Cobalt | Co |
| Chlorine | Cl |
| Chromium | Cr |
| Barium | Ba |
| Beryllium | Be |
| Bromine | Br |
| Magnesium | Mg |
| Manganese | Mn |
| Molybdenum | Mo |
| Titanium | Ti |
| Thallium | Tl |
In some cases, the symbol of the element is derived from the element’s Latin name as shown below:
| Element | Latin name | Symbol |
| Sodium | Natrium | Na |
| Potassium | Kalium | K |
| Copper | Cuprum | Cu |
| Silver | Argentum | Ar |
| Gold | Aurum | Au |
| Lead | Plumbum | Pb |
| Iron | Ferrum | Fe |
| Mercury | Hydragyrum | Hg |
| Tin | Stannum | Sn |
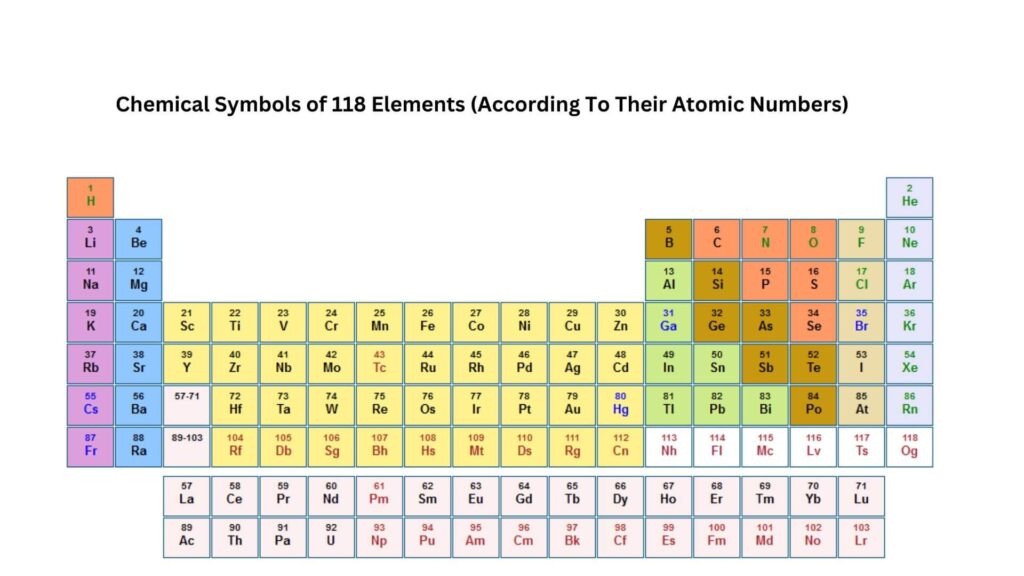
Chemical Symbols of 118 Elements
With the above guide in mind, here are the names and chemical symbols of all 118 elements according to their atomic numbers. For simplicity, we’ve deliberately omitted their Latin names.
*The Atomic Number of an element is the number of protons found in the nucleus of an atom of that element. It uniquely identifies each element and determines its place in the periodic table. We’ll discuss Atomic numbers in detail in the subsequent sections.
| Atomic No. | Name | Symbol |
| 1 | Hydrogen | H |
| 2 | Helium | He |
| 3 | Lithium | Li |
| 4 | Beryllium | Be |
| 5 | Boron | B |
| 6 | Carbon | C |
| 7 | Nitrogen | N |
| 8 | Oxygen | O |
| 9 | Fluorine | F |
| 10 | Neon | Ne |
| 11 | Sodium | Na |
| 12 | Magnesium | Mg |
| 13 | Aluminum | Al |
| 14 | Silicon | Si |
| 15 | Phosphorus | P |
| 16 | Sulfur | S |
| 17 | Chlorine | Cl |
| 18 | Argon | Ar |
| 19 | Potassium | K |
| 20 | Calcium | Ca |
| 21 | Scandium | Sc |
| 22 | Titanium | Ti |
| 23 | Vanadium | V |
| 24 | Chromium | Cr |
| 25 | Manganese | Mn |
| 26 | Iron | Fe |
| 27 | Cobalt | Co |
| 28 | Nickel | Ni |
| 29 | Copper | Cu |
| 30 | Zinc | Zn |
| 31 | Gallium | Ga |
| 32 | Germanium | Ge |
| 33 | Arsenic | As |
| 34 | Selenium | Se |
| 35 | Bromine | Br |
| 36 | Krypton | Kr |
| 37 | Rubidium | Rb |
| 38 | Strontium | Sr |
| 39 | Yttrium | Y |
| 40 | Zirconium | Zr |
| 41 | Niobium | Nb |
| 42 | Molybdenum | Mo |
| 43 | Technetium | Tc |
| 44 | Ruthenium | Ru |
| 45 | Rhodium | Rh |
| 46 | Palladium | Pd |
| 47 | Silver | Ag |
| 48 | Cadmium | Cd |
| 49 | Indium | In |
| 50 | Tin | Sn |
| 51 | Antimony | Sb |
| 52 | Tellurium | Te |
| 53 | Iodine | I |
| 54 | Xenon | Xe |
| 55 | Cesium | Cs |
| 56 | Barium | Ba |
| 57 | Lanthanum | La |
| 58 | Cerium | Ce |
| 59 | Praseodymium | Pr |
| Atomic No. | Name | Symbol |
| 60 | Neodymium | Nd |
| 61 | Promethium | Pm |
| 62 | Samarium | Sm |
| 63 | Europium | Eu |
| 64 | Gadolinium | Gd |
| 65 | Terbium | Tb |
| 66 | Dysprosium | Dy |
| 67 | Holmium | Ho |
| 68 | Erbium | Er |
| 69 | Thulium | Tm |
| 70 | Ytterbium | Yb |
| 71 | Lutetium | Lu |
| 72 | Hafnium | Hf |
| 73 | Tantalum | Ta |
| 74 | Tungsten | W |
| 75 | Rhenium | Re |
| 76 | Osmium | Os |
| 77 | Iridium | Ir |
| 78 | Platinum | Pt |
| 79 | Gold | Au |
| 80 | Mercury | Hg |
| 81 | Thallium | Tl |
| 82 | Lead | Pb |
| 83 | Bismuth | Bi |
| 84 | Polonium | Po |
| 85 | Astatine | At |
| 86 | Radon | Rn |
| 87 | Francium | Fr |
| 88 | Radium | Ra |
| 89 | Actinium | Ac |
| 90 | Thorium | Th |
| 91 | Protactinium | Pa |
| 92 | Uranium | U |
| 93 | Neptunium | Np |
| 94 | Plutonium | Pu |
| 95 | Americium | Am |
| 96 | Curium | Cm |
| 97 | Berkelium | Bk |
| 98 | Californium | Cf |
| 99 | Einsteinium | Es |
| 100 | Fermium | Fm |
| 101 | Mendelevium | Md |
| 102 | Nobelium | No |
| 103 | Lawrencium | Lr |
| 104 | Rutherfordium | Rf |
| 105 | Dubnium | Db |
| 106 | Seaborgium | Sg |
| 107 | Bohrium | Bh |
| 108 | Hassium | Hs |
| 109 | Meitnerium | Mt |
| 110 | Darmstadtium | Ds |
| 111 | Roentgenium | Rg |
| 112 | Copernicium | Cn |
| 113 | Nihonium | Nh |
| 114 | Flerovium | Fl |
| 115 | Moscovium | Mc |
| 116 | Livermorium | Lv |
| 117 | Tennessine | Ts |
| 118 | Oganesson | Og |
NOTE:
I.
Traditionally, when a new element is discovered, the person or group who discovered it gets to name it.
However, until the name is officially approved by IUPAC, the element is given a temporary name based on the Latin words corresponding to its atomic number.
For instance, element 106 was temporarily named unnilhexium (Unh), element 107 was unnilseptium (Uns), and element 108 was unniloctium (Uno) for a few years.
Currently, these elements are renamed after scientists who discovered them (or sometimes places where they were discovered).
For instance, element 106 is now called seaborgium (Sg) in honor of Glenn Seaborg, a Nobel Prize-winning scientist who played a key role in the discovery of numerous heavy elements.
II.
A chemical symbol written without a number indicates that it consists of one atom of the element. For example, H or F represents one atom of hydrogen or fluorine, respectively.
As we have learned in previous posts, two or more atoms may combine to form a molecule. In such cases, the number of atoms in the molecule is represented by a subscript. For example, H2 and F2 represent molecules of hydrogen and fluorine, which consist of two hydrogen and fluorine atoms combined together, respectively.
If the number is written before the chemical symbol, it indicates uncombined atoms or molecules. For instance, 2F represents two uncombined fluorine atoms, and 2F2 represents two fluorine molecules.



