Chromatography is another separation method that can be used to separate two or more solid components of a solution based on their different rates of movement over an absorbent material.
It was originally used to separate colored pigments from plants hence the term chromatography, which is derived from the Greek word “chromos” meaning color. Today, it is used to separate many types of mixtures—not only colored pigments.
There are several types of chromatography, but for the purpose of this post, which aims to introduce you to chromatography, we will focus on the simplest type called paper chromatography.
Other types of chromatography include liquid chromatography, gas chromatography, ion-exchange chromatography, and affinity chromatography.
Paper Chromatography
Before we discuss what paper chromatography is or how it works, let’s first familiarize ourselves with two major terms that are commonly used in chromatography discourse at large.
- Stationary Phase: The stationary phase is a solid or immobile liquid that does not move during the chromatographic process. It is typically a solid support material such as paper, silica gel, or a polymer coated on a solid support.
- Mobile Phase: The mobile phase is a fluid that moves through or over the stationary phase, carrying the sample components with it. It can be a liquid or a gas, depending on the type of chromatography being performed (liquid chromatography or gas chromatography).
The separation process in chromatography is based on the interaction between the components of the mixture to be separated and the two phases: the “stationary phase” and “mobile phase.”
Generally, paper chromatography involves two major processes taking place simultaneously. These include solubility, which is the tendency of a substance to dissolve in a solvent, and adsorption, which is the tendency of a substance to stick to an adsorbent material.
If we are to use the stationary phase and mobile phase terminologies, we can say solubility is how well a component dissolves in the mobile phase while adsorption is how well a component sticks to the stationary phase.
The mixture to be separated (or analyte) is introduced to the mobile phase and as it moves over the stationary phase, its components interact with both of these phases. Depending on their adsorption and solubility, some components will interact more strongly (or stick) to the stationary phase and their movement over this phase will be slowed down. Conversely, other components will interact less strongly (not stick) over the stationary phase and pass over it quickly. (Such components can also be said to interact strongly with the mobile phase). It is these differences in interactions with the two phases that bring about separation of the different components of the mixture. And this is the main principle of chromatography.
How Paper Chromatography Works
- To perform paper chromatography, you need the above-mentioned two phases: a stationary phase and a mobile phase.
- A piece of special paper, typically filter paper or chromatography paper, is often used as the stationary phase.
- The mobile phase is usually a solvent or a suitable fluid that can move through or over the stationary phase, carrying the sample components with it.
- The sample containing the mixture of components to be separated is spotted or applied near the bottom of the paper, usually as a small dot.
- The bottom of the paper containing the sample is placed in a container with a solvent (the mobile phase).
- The solvent moves up the paper through capillary action, carrying the components of the mixture along with it.
- As the solvent moves up the paper, it interacts differently with each component of the sample mixture.
- Components with higher affinity for the solvent (more soluble and less sticky) will move more quickly up the paper, while those with lower affinity will move more slowly (less soluble and stickier).
- This results in the separation of the components into distinct bands or spots along the paper.
Here is a simple experiment to demonstrate how this works.
Separating Components of Black Ink Using Paper Chromatography
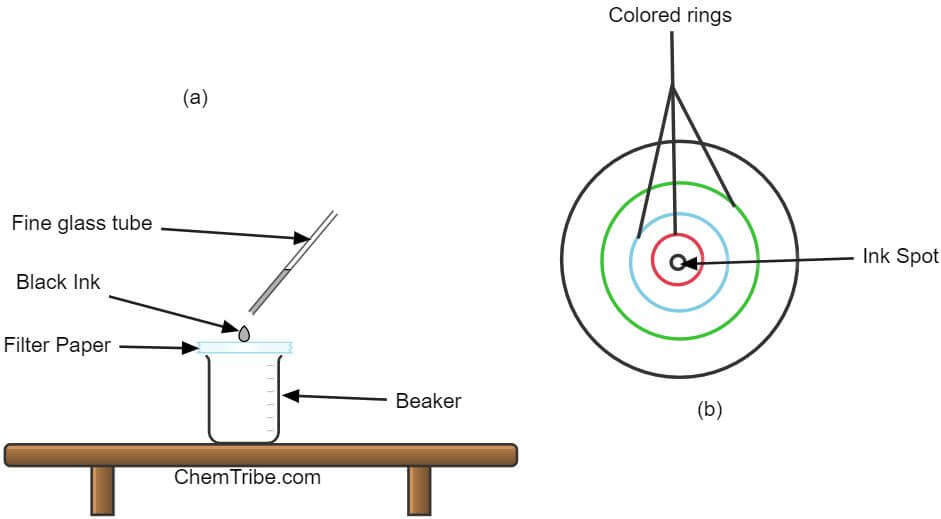
Apparatus and chemicals needed
- Beaker
- Fine Glass Tube
- Teat pipette
- Filter paper
- Propanone
- Black Ink
Procedure
- Place a circular piece of filter paper over a beaker.
- Put a small drop of black ink in the middle of the filter paper using a fine glass tube.
- Leave the drop to dry and apply another drop on the same spot. Apply 4-5 more drops ensuring that the spot doesn’t get too large.
- Using a teat pipette, put a drop of propanone (acetate) over the spot where you put the black ink.
- Once the solvent dries, add several more drops of propanone, allowing each drop to spread and dry before putting the next.
- Continue adding more drops of the solvent until it spreads close to the edge of the paper.
Observation and Inferences
Red, blue and yellow-colored rings are formed at increasing distances from the centre of the filter paper.
Black ink is a mixture of red, blue, and yellow dyes. When a solvent is added, these dyes dissolve. As the solvent spreads outwards, it carries with it these dissolved dyes. At the same time, the dyes stick to the filter paper (adsorbent material), slowing down their movements.
The speed at which each dye moves depends on its solubility in the solvent (propanone) and its stickiness to the filter paper. The most soluble and least sticky dye moves fastest. Those that are less soluble and stickier move slowly. Consequently, each dye moves at its own pace leading to separation. Each dye forms a colored ring away from the point of application.
Identifying the Components of a Mixture
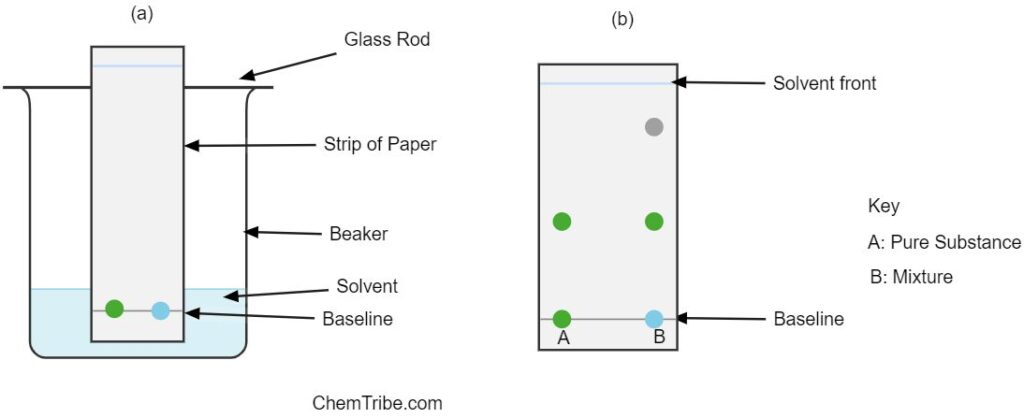
Besides separation, chromatography can also be used to identify the components of a mixture. This is done by spotting a known substance along the unknown or suspect mixture as shown in the figure above. The paper strip is then placed in a beaker containing an appropriate solvent as shown in Figure (a). The solvent is allowed to ascend to the top (solvent front) and the paper is allowed to dry. The position of the spots from the suspect mixture and the pure substance are noted and compared.
If any of the spots in the unknown mixture moves the same distance as the spot in a known or pure substance, then it is concluded that the mixture contains the pure substance as one of the components. Figure (b) shows a sample chromatogram.
Ascending and Descending Paper Chromatography
The above procedure is known as ascending paper chromatography because the solvent (mobile phase) moves upwards by capillary action, carrying the sample components with it.
The opposite of ascending chromatography is descending paper chromatography and it entails the solvent moving down the chromatography paper as shown in the figure below.
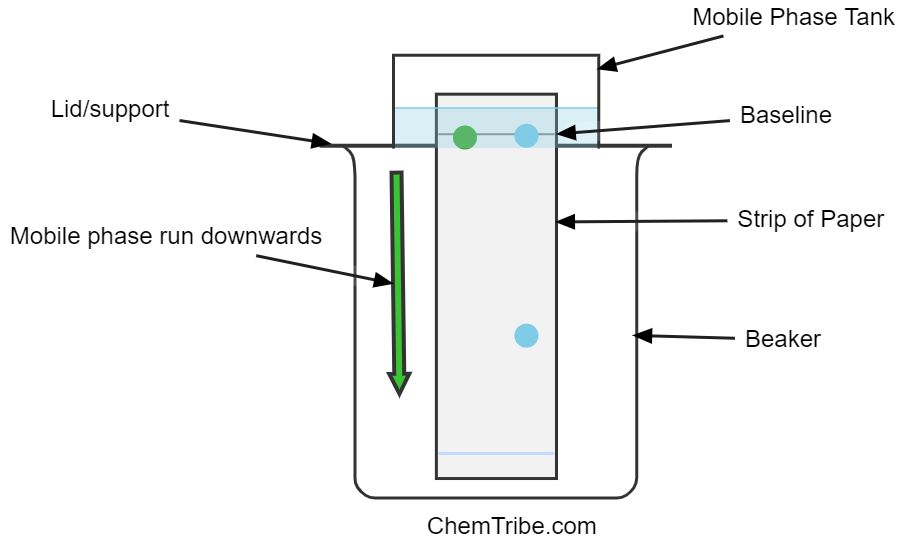
The sample is typically applied at the top of the chromatography paper, and the solvent is allowed to flow downwards.
As the solvent progresses downwards, it carries the sample components along, and separation occurs based on the differential interactions between the components and the stationary and mobile phases.
Paper Chromatography Is Also Used To Analyze Colorless Substances
As we mentioned in the introduction, chromatography was originally used for the separation of colored substances, which could be identified easily. Today, however, it is used for the separation and identification of a large variety of substances, which are not necessarily colored. Examples include sugars, amino acids, and steroids. Various methods are used for the identification of these substances. For instance, the chromatogram may be sprayed with reagents that interact with the separated substances to produce colored spots. Alternatively, the chromatogram may be exposed to ultraviolet light, which causes various substances to fluoresce making them visible.
Application of Paper Chromatography
- To detect the presence of impurities in substances. In the absence of impurities, a substance will only give one spot when run in different solvents. The presence of impurities is indicated by the presence of one or more additional spots.
- To detect the presence of additives in commercially processed foods.
- To separate and identify pigments in plants, such as chlorophyll, carotenoids, and anthocyanins.
- In sports, it can be used to test the presence of illegal substances (like steroids) in the urine and blood samples of contestants.
- In biochemistry, to separate and quantify amino acids present in biological samples.
- In forensic science and pharmaceutical analysis, chromatography is used for the detection and separation of drugs and their metabolites.
Recap of Key Terms
- Stationary Phase: The medium (paper) in which the sample components are separated.
- Mobile Phase: The solvent or mixture of solvents that moves through the stationary phase.
- Adsorption: The process by which the sample components adhere to the surface of the stationary phase.
- Solubility: The ability of a substance to dissolve in a solvent.
- Solvent: The solvent is the liquid used as the mobile phase in chromatography.
- Analyte: The substance or mixture of substances being analyzed in chromatography.
- Baseline: The baseline is the starting line on the chromatogram where the sample is applied.
- Solvent Front: The leading edge of the mobile phase as it moves through the stationary phase.
- Chromatogram: The output of a chromatography run or the visual representation of the separation process that has occurred during chromatography.