After learning about different techniques that we can use to separate mixtures into their components, it is necessary to understand how to determine whether the components or a substance we are interested in are pure or impure. The best way to do this is by analyzing one or more of its properties. These properties include densities, melting points, and boiling points.
In chemistry, the most commonly measured properties for determining the purity of a substance are its melting point and boiling point.
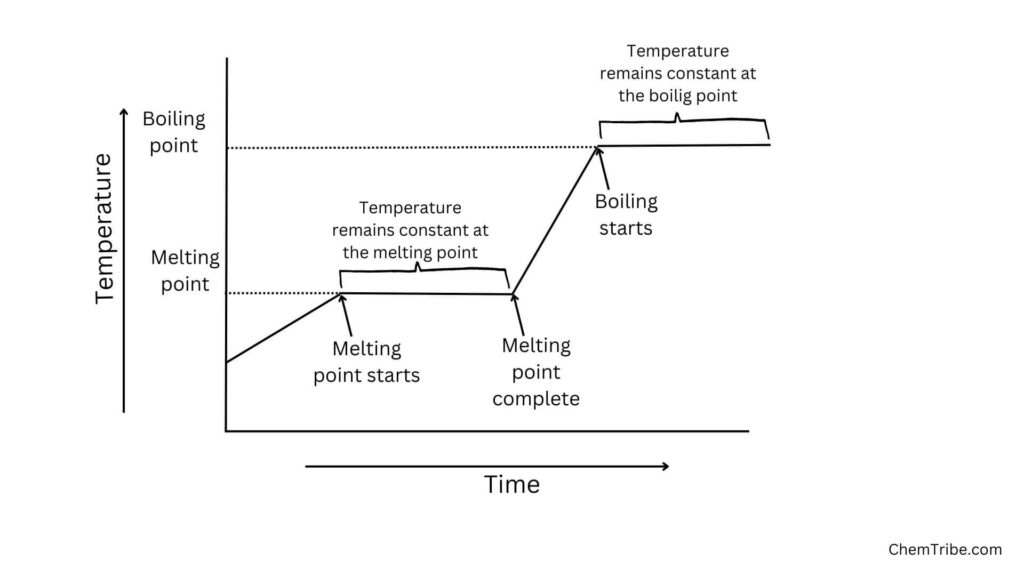
When a pure substance in its solid state is heated it starts, it starts melting at a definite temperature. The temperature remains constant as long as the solid remains (figure below). Only after all the solid has melted does the temperature start to rise. If heating is continued, the liquid starts to boil at characteristic temperature. The temperature remains constant as long as any liquid remains.
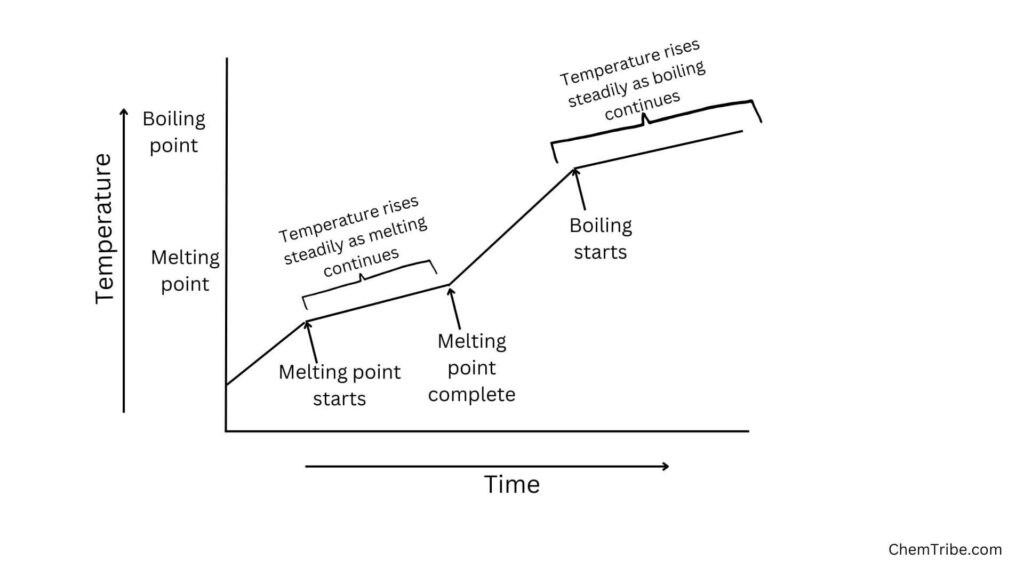
When an impure substance in its solid state is heated, it starts melting at a temperature below that of a pure substance. The temperature doesn’t remain constant but rises steadily as the melting point continues. After all the solid has melted, the temperature starts to rise much faster. Boiling starts at a higher temperature than that of a pure substance. The temperature doesn’t remain constant but rises as boiling continues.
Effect of Impurities on Melting Point
Pure substances melt sharply at a definite temperature. The presence of impurities lowers the melting point and widens the temperature range within which the substance melts. The more the amount of impurities, the lower the melting point falls.
The reason why impurities lower melting points can be explained as follows:
In a pure substance, the molecules are arranged uniformly. When heat is applied, these molecules absorb energy and vibrate until they overcome the forces holding them together, resulting in melting at a specific temperature. The melting point of a pure substance is, therefore, sharp and well-defined because all molecules melt at the same temperature.
When impurities are present in a substance, they interfere with the uniform arrangement of molecules in the crystal lattice. This interference introduces defects in the crystal lattice or weakens the forces between the molecules, making it easier for them to break apart and melt. As a result, the melting point of an impure substance is lower than that of a pure substance, and the melting temperature range is broader.
Effect of Impurities on Boiling Point
Similar to the melting point, the boiling point of pure substances occurs at a specific temperature, and the transition from liquid to gas phase happens sharply. However, the presence of impurities increases the boiling point and broadens the boiling temperature range.
The main reason why impurities elevate boiling points and broaden the boiling temperature range is similar to their effect on lowering melting points: they disrupt the uniform molecular arrangement within the substance.
We know you are probably asking: Why do impurities lower the melting point yet increase the boiling point? Well, here is a quick explanation:
As mentioned above, impurities introduce defects in the crystal lattice structure of a substance, making it easier for the molecules to break free and transition to the liquid phase. As a result, the melting point decreases because less energy is required to disrupt the crystal lattice and initiate melting.
When it comes to boiling point, things are a bit different due to the difference in the nature of the phase transition. In the liquid phase, molecules are not arranged in a regular lattice structure like in the solid phase. So, impurities don’t disrupt the uniformity of crystal lattice structure like in the solid phase but disrupt the number of intermolecular forces within the liquid. In particular, they introduce additional intermolecular forces within the liquid. These additional intermolecular forces must be overcome for the liquid to transition to the gas phase. As a result, the boiling point increases because more energy is required to overcome extra intermolecular forces to achieve vaporization.
The melting point and boiling point of a substance can therefore serve as indicators of its purity. A sharp, well-defined melting or boiling point indicates high purity, while deviations from the expected melting and boiling points or broadened temperature ranges suggest the presence of impurities.
Practical Applications of the Alterations of Melting Points and Boling Points of Substances by the Presence of Impurities
Applications of Lowering Of Melting Point by Impurities
The lowering of melting points by impurities is applied in various ways:
- During winter in temperate countries, salt is placed on roads where it mixes with snow. This lowers the melting point of ice ensuring that the roads are free of snow temperatures below 00C. Depending on the amount of salt added, the melting point of ice can be lowered to -180C.
- It is also used in the extraction of metals. For instance, during the extraction of sodium by electrolysis of molten sodium chloride, a little sodium chloride is added. This lowers the melting point of sodium chloride from 8010C to 6000C. This makes the process economical as it is very expensive to heat pure sodium chloride to its melting point.
- To create automobile antifreeze solutions, impurities such as ethylene glycol are added to water to lower its freezing point. This is used in automobile cooling systems to prevent engine coolant from freezing in cold temperatures, thereby protecting the engine from damage.
- The addition of salt to ice in ice cream makers lowers the freezing point of the ice, allowing for faster freezing of the ice cream mixture. This results in a smoother and creamier ice cream texture.
Applications of Elevating Of Boiling Point by Impurities
There are also applications where increasing the boiling point of a substance by impurities can be beneficial:
- In thermal fluid systems and heat transfer applications, impurities can be added to fluids to increase their boiling points and enhance their thermal stability. This is important for maintaining consistent operating temperatures and preventing the formation of vapor bubbles or boiling in the system.
- In some cooling systems, such as those used in high-performance engines or electronics, additives are incorporated into coolant solutions to raise their boiling points. This helps prevent overheating and ensures efficient heat dissipation in demanding operating conditions.



