Key Takeaways:
- Distillation is a separation technique used to separate components within a liquid mixture based on differences in their boiling points.
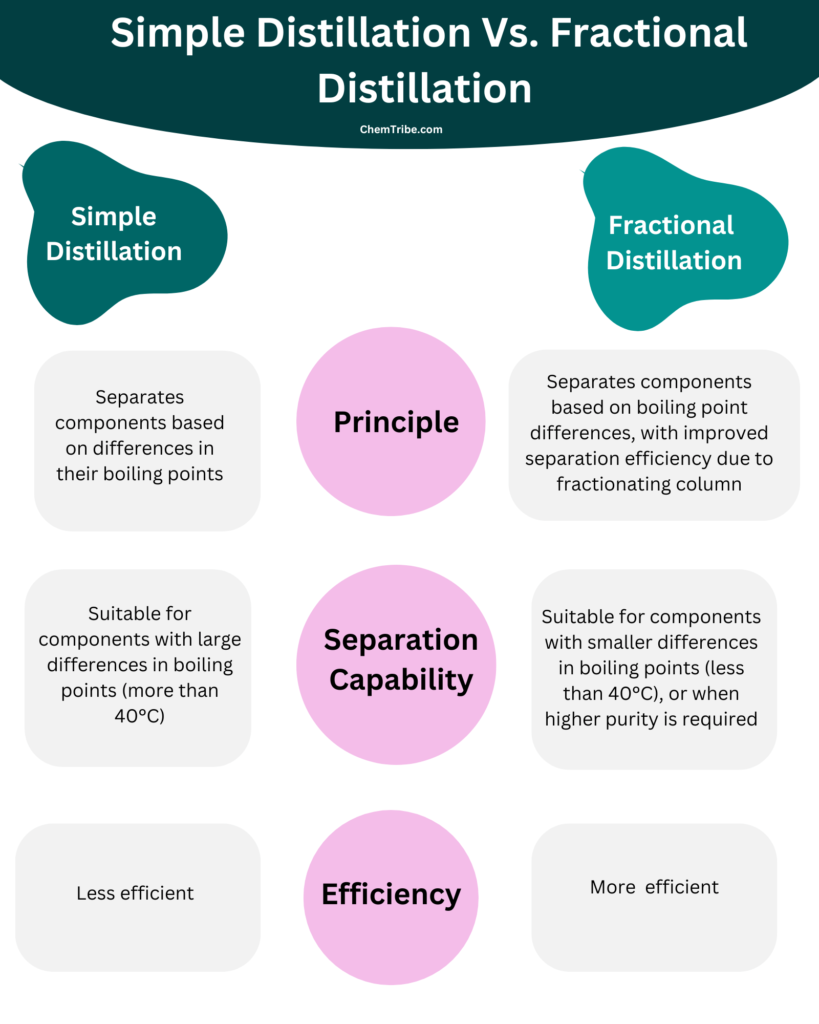
- There are two main types of distillation: simple distillation and fractional distillation.
- Simple distillation is the most basic form of distillation and is used to separate components with large differences in boiling points (more than 400C).
- Fractional distillation is more efficient than simple distillation and is used to separate components with smaller differences in boiling points (less than 400C), or when higher purity is required.
Distillation is a separation technique that’s commonly used to separate components of miscible solutions by exploiting differences in their boiling points. It involves heating a liquid mixture to its boiling point to vaporize the more volatile components and then cooling the vapor to condense it back into liquid form. The condensed liquid, known as the distillate, is collected separately from the original mixture.
When a substance is heated to its boiling point, it is converted to vapor and is then said to have vaporized. Substances with low boiling points tend to vaporize fast and are said to be volatile. And those with higher boiling points don’t vaporize easily and are considered to be non-volatile. It is this disparity in volatility that chemists exploit during the distillation process to separate various components based on their differences in boiling points.
There are two main types of distillation: simple distillation and fractional distillation.
1. Simple Distillation
Simple distillation is the most basic form of distillation. The liquid mixture to be separated is heated to its boiling point, causing the more volatile components to vaporize. The vapors are then cooled and condensed back into liquid form or distillate.
Here is a setup that can be used to illustrate a simple distillation process.
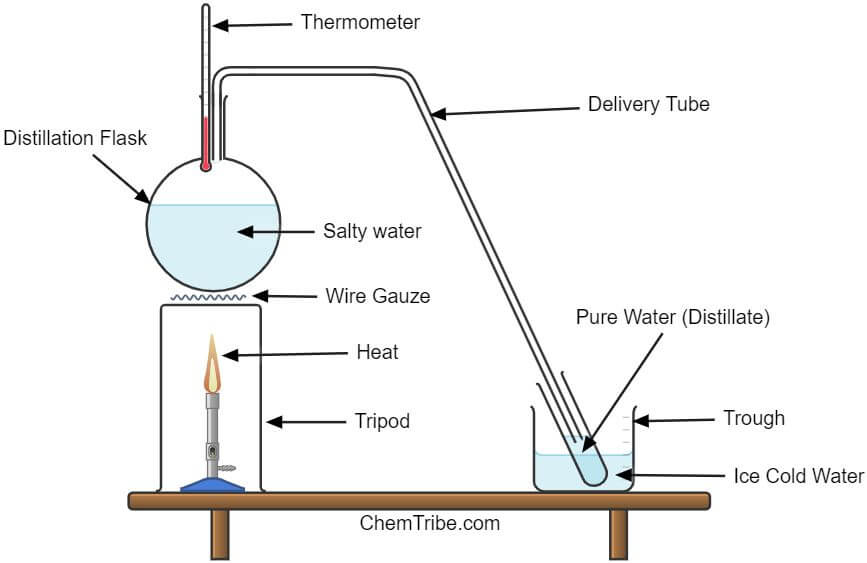
In the above setup, the solution (a mixture of common salt and water) is boiled in a distillation flask. As a result, liquid water is converted to vapor. However, instead of allowing the water vapor to escape into the air (like we saw in evaporation), it is passed into a test tube that is surrounded by ice-cold water.
The water vapor is cooled and converted back into liquid water, a process called condensation.
The liquid collected in the test tube is commonly referred to as the distillate. It is mainly composed of water.
Salt is left as a residue in the distillation flask after all the water has evaporated.
Improving the Condensation Process with a Liebig Condenser
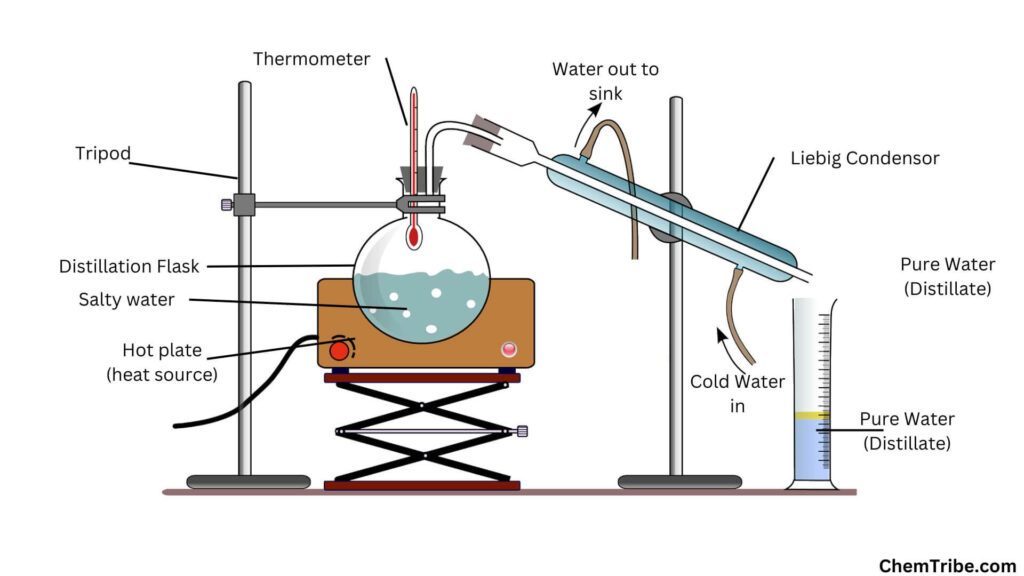
Instead of the delivery tube above, an improved apparatus for simple distillation often incorporates a Liebig condenser. A Liebig condenser is made up of a vessel in which cold water is passed. A tube carrying the water vapor runs through the middle of the condenser. The cold water cools the vapor turning into liquid water. The liquid water is collected as distillate in a container.
A Liebig condenser uses a counterflow principle to cool the vapor efficiently. Cold water flows in the direction opposite to the flow of the water vapor. This creates a permanent cold surface, onto which water vapor condenses.
The counterflow principle is a fluid flow pattern where two fluids (or a fluid and a solid) move in opposite directions relative to each other. It is commonly used in various engineering and industrial processes, including heat exchangers, where it allows for efficient heat transfer between two fluids. By having the fluids flow in opposite directions, the counterflow arrangement maximizes the temperature gradient between them, resulting in more effective heat exchange compared to parallel flow arrangements.
It is important to note that if the inlet and out of the Liebig condenser were to be exchanged, condensation would still take place but it would be less efficient.
Main Advantages of Using a Liebig Condenser in a Simple Distillation Process
- More Efficient: The Liebig condenser provides efficient cooling of the vapor, which allows it to condense back into liquid form quickly. This helps to maximize the yield of the desired distillate.
- High Surface Area for Condensation: The design of the Liebig condenser provides a large surface area for the vapor to come into contact with the cooling water. This enhances the condensation process and ensures effective heat transfer.
- More Resistant to Thermal Shock: Made of sturdy borosilicate glass, Liebig condensers are durable and resistant to thermal shock. This makes them suitable for use in high-temperature distillation processes without the risk of breakage.
Separation of Miscible Liquids
The above setup is a perfect example of how simple distillation can be used to separate a dissolved solute (common salt) from its solvent (water). But simple distillation can also be used to separate a mixture of two miscible liquids with different boiling points. For instance, it is usually applied to separate a mixture of water (boiling point of 1000C) and ethanol (boiling point of 780C).
Here is how the process works:
The mixture is heated gently. It begins to boil somewhere between 780C and 1000C depending on the proportion of water and ethanol in the mixture. The vapor formed has a higher concentration of ethanol than the original mixture. It is condensed into a distillate, which is collected in a separate container. As hinted, the distillate is richer in ethanol than the original mixture. If the distillate is re-distilled, the concentration of ethanol increases. If the distillation process is repeated a few more times, the distillate collected is almost pure ethanol.
As you might anticipate, this procedure can be quite time-consuming. This leads us to the second, and more efficient, method of distillation: Fractional distillation.
2. Fractional Distillation
Fractional distillation is more efficient than simple distillation. That’s why simple distillation is typically used when the boiling points of the components to be separated differ significantly (usually more than 40°C) and when there is no risk of decomposition or significant chemical reaction occurring at the boiling temperature.
In the case of separating a mixture of water and ethanol, for instance, it has been proven to be more efficient and faster.
What brings this efficiency is the addition of a Fractionating Column which is often placed above the distillation flask. The column is packed with glass beads to provide a large surface area over which some of the vapor condenses before passing into the Liebig condenser.
The role of the fractionating column is to allow the vapor of the less volatile liquid to condense and flow back into the flask before its boiling point is reached.
Fractional distillation is often used to separate miscible liquids that have different but close boiling points.
Separating Ethanol and Water Mixture Using Fractional Distillation
The setup is comprised of a fractionating column as shown below.
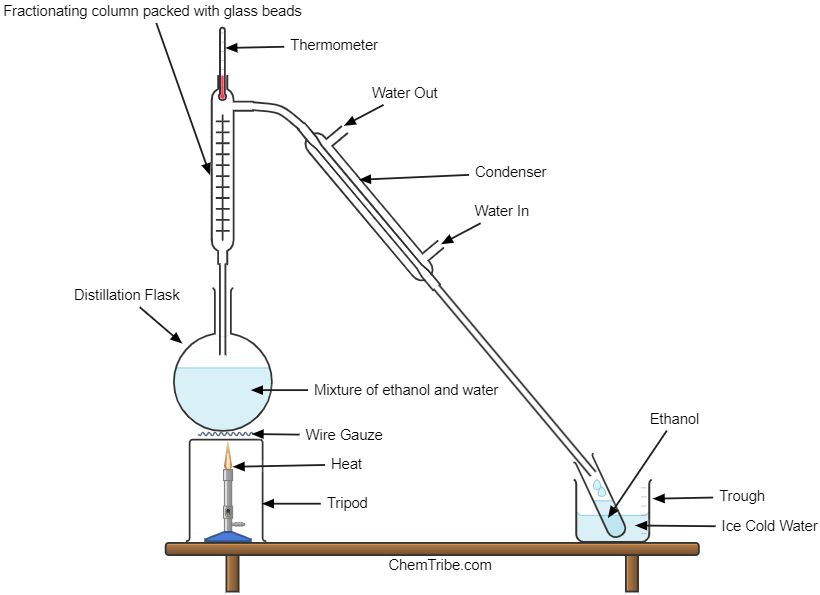
On heating the mixture, it boils at a temperature somewhere between 780C and 1000C depending on the proportion of water and ethanol in the mixture. The vapor formed has a higher concentration of ethanol than the original mixture.
The temperature of the fractionating column is higher at the bottom than at the top. So, as the vapor rises up the column, the less volatile component (one with a higher boiling point, which is water) condenses on the beads and flows back into the distillation flask. The vapor of the more volatile component (the one with the lower boiling point, which is ethanol) passes into the Liebig condenser where it is condensed into a liquid. It is then collected into another container.
When all the volatile component (ethanol) has distilled out, the thermometer reading rises to about 1000C which indicates that it is now the less volatile liquid (water) that’s distilling out. At this stage, the distillation flask is mainly composed of water and the process is discontinued.
Limitations of Fractional Distillation
While fractional distillation is more efficient than simple distillation, it has a few limitations, including:
- Fractional distillation is less effective when separating components with boiling points that are very close together.
- While fractional distillation can achieve high levels of purity, it may not COMPLETELY separate the components of a mixture. This may result in some overlap or contamination between components, leading to impurities in the final products. This limitation can be particularly problematic in separation processes or industries where high purity is essential, such as pharmaceutical industries. In such cases, additional purification steps or alternative separation techniques may be required to obtain the desired level of purity.
- Some mixtures may not be effectively separated using fractional distillation due chemical reactions which may take place during the distillation process. Some mixtures may undergo chemical reactions during heating, resulting in the formation of new compounds or decomposition products. These chemical changes can interfere with the separation process and may necessitate further purification.
Simple Distillation Vs. Fractional Distillation Summary