Answer
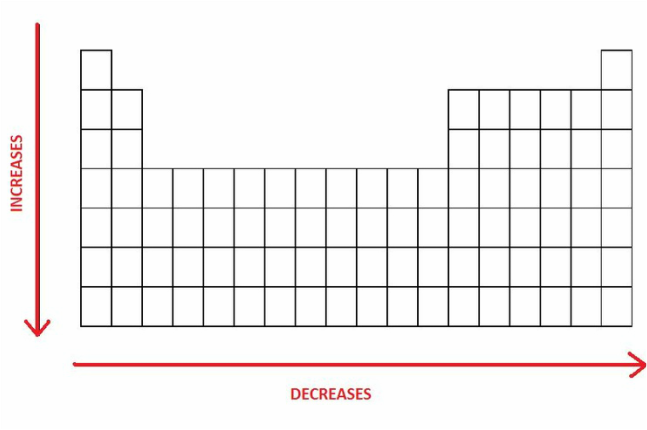
No. Atomic radii DECREASE as we move from left to right across a period in the periodic table. The table below, for example, shows the atomic radii of Period 3 elements—you can observe a steady decrease from sodium (Na) to argon (Ar) through magnesium (Mg), aluminum (Al), silicon (Si), phosphorus (P), sulfur (S), and chlorine (Cl).
| Element | Atomic Radius (approx. nm) |
|---|---|
| Sodium (Na) | 0.191 |
| Magnesium (Mg) | 0.160 |
| Aluminum (Al) | 0.130 |
| Silicon (Si) | 0.118 |
| Phosphorus (P) | 0.110 |
| Sulfur (S) | 0.102 |
| Chlorine (Cl) | 0.099 |
| Argon (Ar) | 0.095 |
Explanation
The decrease in atomic radii from left to right across a period is due to the increase in nuclear charge. As the atomic number increases from sodium to argon, more protons are added to the nucleus, while the added electrons occupy the same principal energy level. Because electrons within the same shell do not effectively shield one another from the nucleus, the growing positive charge is not offset by the additional electrons. This results in a stronger attraction between the nucleus and the electrons, pulling them closer and thereby reducing the size of the atom.