Evaporation is the process through which liquid changes into vapor when heated. (The term vapor denotes a gas that can be condensed easily to liquid). It is one of the basic separation techniques in chemistry, which is majorly used to separate soluble solids from their solvents.
For example, sodium chloride (common salt) readily dissolves in water, making it impractical to separate using methods such as decantation and filtration, which we discussed earlier.
You need to boil such a solution to evaporate the solvent (water) leaving the solute behind (sodium chloride).
Evaporation is a separation method that involves the conversion of a liquid into its gaseous state by heating or exposure to air. During evaporation, the more volatile components of a mixture vaporize and escape into the atmosphere, leaving behind the less volatile components as residue.
Here is a simple experimental setup to illustrate evaporation as a separation technique in chemistry:
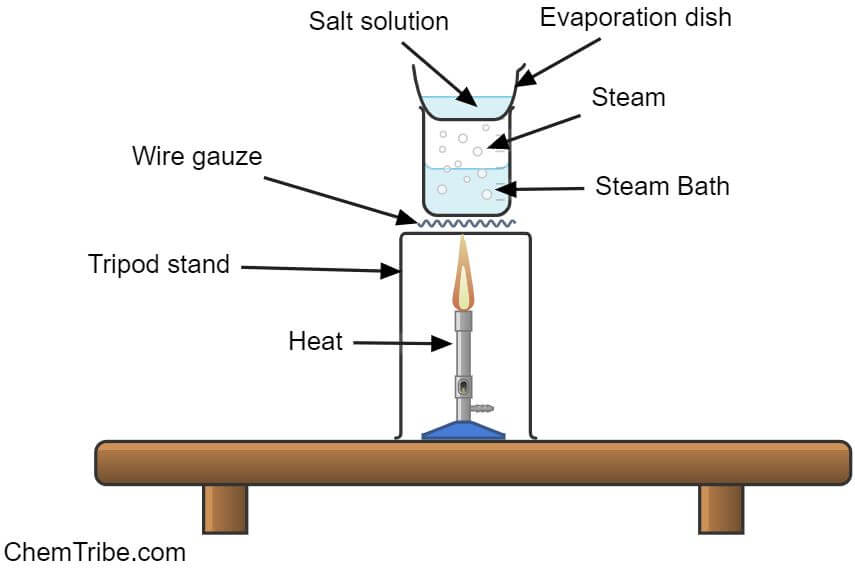
Apparatus and Chemicals Needed
- Bunsen burner
- Beaker
- Wire gauze
- Tripod Stand
- Evaporating dish
- Sodium chloride (Common Salt)
Procedure
- Make a solution of sodium chloride (salt) and water.
- Transfer the solution to an evaporating dish.
- Heat the solution over a steam bath as shown in the figure above.
Observation and Discussion
Water evaporates leaving sodium chloride (salt) in the evaporating dish. This process is called evaporation. It is the same process that is used to obtain salt from seawater.
NOTE: The solution was heated over a steam bath to ensure that the salt crystals are not heated directly. This prevents “splitting”, which refers to the jumping out of overheated salt crystals.
Condensation
Condensation is the opposite of evaporation. Essentially, it is the conversion of a vapor or gas into a liquid by cooling.
Condensation is not typically considered a separation technique in the same sense as methods we’ve discussed previously like decantation or filtration. Instead, condensation is a phenomenon that occurs during certain separation processes, particularly those involving the transformation of gases or vapors into liquids.
A perfect example of a separation technique where condensation plays a crucial role is distillation. During distillation, a mixture is heated to separate its components based on differences in their boiling points. The vaporized components are then condensed back into liquid form by cooling, usually through the use of a condenser. The liquid with a higher boiling point condenses first and is collected. The liquid with the lower boiling point is collected last.
This condensation step allows for the separation and collection of the purified components.