The world would really be boring if each substance existed on its own. Fortunately, substances combine to form mixtures. This implies that mixtures are everywhere around us:
- Rocks are mixtures of minerals
- Air is a mixture of gases
- Oceans and lakes are mixtures of water, minerals, and gases
- A salad is a mixture of different ingredients
Basically, if two or more substances (elements or compounds or both) are combined in any proportion, such that they don’t undergo any chemical change but retain their individual properties, the resulting substance is called a mixture.
Mixtures can be categorized into two main categories with a few exceptions: homogenous and heterogeneous mixtures.
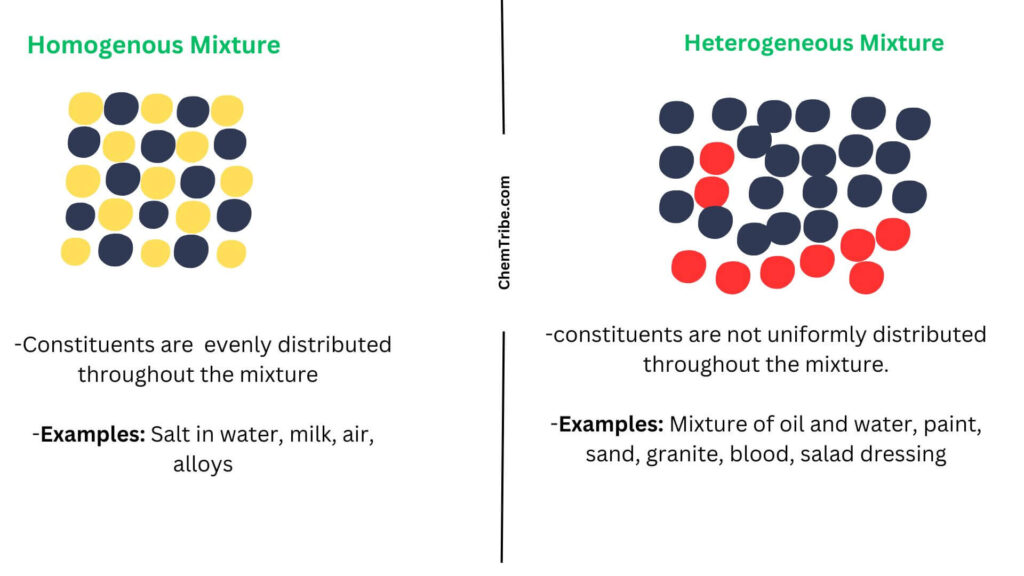
Homogenous Mixtures
These are mixtures whose constituents are mixed uniformly or evenly distributed throughout the mixture at a molecular level. This means that the mixture has a uniform appearance and properties throughout.
Examples:
- Mixing sugar in water: The dissolved sugar spreads out evenly throughout the water and if you sample any part of the solution, you’ll find the same amount of sugar.
- Milk: Contains proteins, sugar, water, and fat but all these components cannot be easily separated or tasted individually.
- Air: The components (mixture of gases) are uniformly distributed.
- Alloys (Bronze or steel): Don’t have distinguishable regions of each component
Heterogeneous mixtures
These are mixtures whose components are not uniformly distributed throughout the mixture. Instead, the mixture contains distinct regions with different compositions. They often have visible boundaries between their components.
Examples:
- Suspension: One part is always a liquid while the other is a solid
- Salad dressings: They always contain oil and vinegar, which don’t mix
- A chocolate chip cookie: If you took samples of the cookie, they would be different from each other. Some would have chocolate chips while others wouldn’t.
- Paints: contain solid pigments, color, and liquid
- A mixture of oil and water: oil forms separate droplets within the water
- Sand: Consists of different sizes and types of particles
- Blood: Consists of different components, such as white blood cells, red blood cells, platelets, and plasma, which are not uniformly distributed.
- Granite: Composed of grains of different minerals, such as quartz, mica, and feldspar.
Deceptive Homogeneity
Some heterogeneous mixtures appear to be homogenous. For instance, a patch of soil may appear to be uniform. But soil is a heterogeneous mixture because each soil sample that you analyze may contain different amounts of rocks, minerals, and other substances.
A helpful hint to distinguish between homogeneous and heterogeneous mixtures is to consider sampling the mixture. If you can observe more than one phase of matter or different regions within your samples, it is likely a heterogeneous mixture. On the other hand, if the composition of the mixture appears uniform regardless of where you sample it, it is likely a homogeneous mixture.
Homogeneous Mixtures vs Solutions
Homogeneous mixtures and solutions are often used interchangeably, but there’s a slight distinction.
Homogeneous mixtures refer to any mixture where the components are uniformly distributed throughout the mixture and are indistinguishable from one another, regardless of whether they are in the same phase.
Solutions, on the other hand, specifically refer to homogeneous mixtures where a solute is dissolved in a solvent, typically forming a liquid phase.
So, while all solutions are homogeneous mixtures, not all homogeneous mixtures are solutions.



