The primary states of matter—solid, liquid, and gas—exhibit distinct properties, which we have explored in this post.
Now, let’s revisit the properties of gases.
We previously established that “Gases have indefinite shapes and volumes but definite mass.”
While we explained why gases have indefinite shapes and volumes, we only briefly mentioned that they have definite mass.
If this left you with any questions or doubts, rest assured, we will not only explain it in this post but also demonstrate that gases do indeed have mass.
Let’s get started.
Materials Needed
- Two Plastic footballs
- A Piece of straight, light stick
- Pieces of string
- A pump
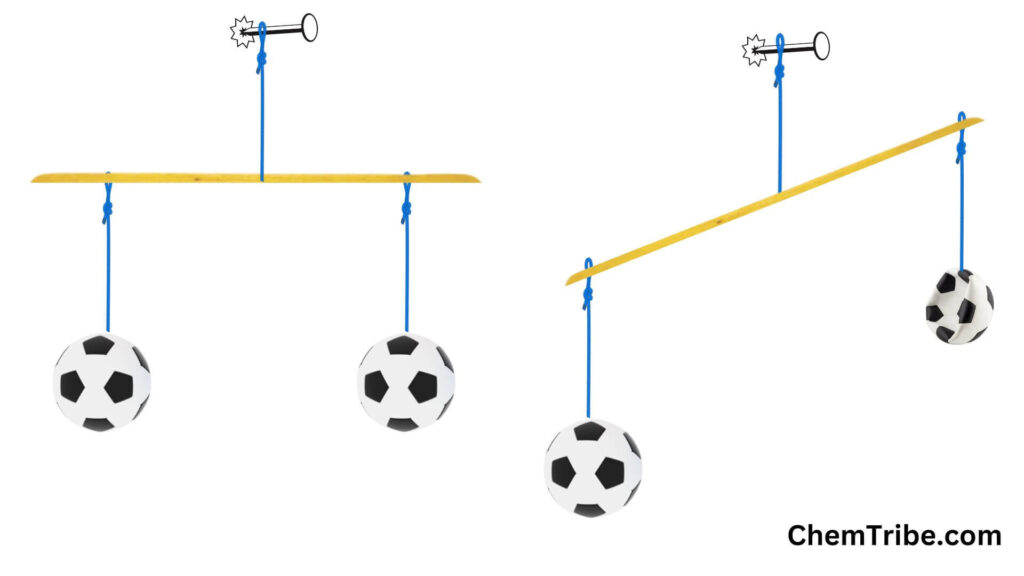
Procedure
- Suspend the straight stick using a string to balance it on both sides.
- Inflate both plastic footballs using the pump until they are the same size.
- Hang each ball with a string on opposite sides of the stick.
- Deflate one of the plastic footballs and observe the outcome.
Observation and Discussion
When you deflate one of the balls, the two balls no longer balance, causing the stick to tilt toward the side with the inflated ball (see the above figure). This clearly indicates that the inflated ball is heavier. Since we stated that mass is what gives an object its weight, this brief experiment confirms that gases do indeed have mass. However, it’s important to note that gases have significantly less mass compared to solids and liquids. This explains why gases are very light.



