Copper sulphate, also known as cupric sulphate or copper (II) sulphate, is a chemical compound commonly used in chemistry experiments and in various industries. When dissolved in water, it forms a distinct blue solution.
However, there are times when we need to extract pure copper sulfate from this solution, for example, in laboratory research, industrial manufacturing, and educational demonstrations. How do we proceed in such cases? How can you separate copper sulfate from its aqueous solution?
Well, one effective method is through a technique we discussed earlier: Crystallization.
For people who are probably hearing this separation technique for the first time, crystallization is used to separate substances by inducing the formation of solid crystals from a solution. A solution containing the dissolved substance is allowed to cool or evaporate slowly under controlled conditions. As the temperature decreases or the solvent evaporates, the solubility of the solute decreases, leading to the formation of crystals. These crystals can be separated from the remaining solution by filtration or centrifugation, resulting in the isolation of the pure substance.
Here’s a quick experiment to demonstrate how crystallization can be used to obtain copper sulfate crystals from an aqueous copper sulfate solution.
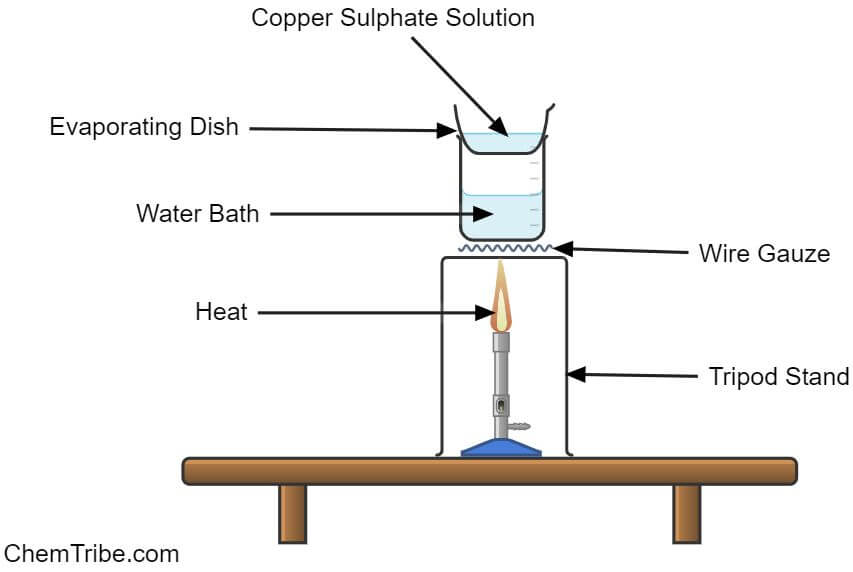
Apparatus and Chemicals Needed
- Bunsen burner
- Beaker
- Wire gauze
- Tripod Stand
- Evaporating dish
- Beaker
- Glass Rod
- Copper sulphate
Procedure
- Put 10cm3 of concentrated copper (II) sulphate solution into an evaporating dish.
- Arrange the apparatus as shown in the above figure.
- Using a water bath, heat the solution to evaporate excess water.
- As heating continues, dip a glass rod into the solution regularly and allow it to cool in the air.
- When crystals start forming on the glass rod, remove the evaporating dish from the water bath and allow it to cool.
Observation and Discussion
Upon cooling, blue crystals form on the evaporating dish, indicating that crystallization has occurred. With this simple process, we have successfully obtained copper sulfate crystals from an aqueous copper sulfate solution. This is an example of evaporative crystallization.
The glass rod was dipped into the solution to confirm if it was saturated and capable of forming crystals upon cooling. If crystals form on the glass rod, it indicates that the solution is saturated (ready to form crystals). A saturated solution is one in which no more solute can dissolve at a given temperature.



