In the previous section, we’ve learnt that a compound is a pure substance that is made up of two or more elements chemically combined. We also learnt in previous sections that that matter exists as pure substances and mixtures.
So, what is the difference between a mixture and a compound?
Well, the difference between a mixture and a compound can become clearer when we consider the scenario of combining iron fillings and sulfur: imagine grinding them together and attempting to separate them using a magnet. Then, heating the mixture strongly and again attempting to separate the new substance formed using a magnet.
When you try to septate the mixture with a magnet before heating them, the iron fillings are attracted to the magnet, making it easy to separate iron from Sulphur.
On heating, a black solid is formed, which cannot be separated into its constituents using a magnet.
The results indicate that before the iron-Sulphur mixture was heated, its components (iron and Sulphur) retained their properties. That’s why we could take advantage of iron’s magnetic property to separate the mixture.
When the mixture is heated, iron and Sulphur combine to form a new substance with unique properties that are different from those or iron and Sulphur. The new substance is Iron (II) sulphide.
We know that Iron and Sulphur are elements. Before heating, we had simply a mixture of two elements, with each having its own unique properties.
On heating, Iron and Sulphur particles combine chemically to form a new substance with totally different properties. The two elements are now bound together by a chemical bond, which is so strong and would require an input of significant amount of energy to break and separate the two elements.
The Iron (II) sulphide is a compound, which has been formed by chemically combining two elements. Iron and Sulphur are now chemically bonded and cannot be separated by a magnet.
Distinct Formation Characteristics
The formation of the new substance (compound) has certain features that are very distinct from the formation of a mixture. These include:
- Heat change is involved. Unlike obtaining a mixture, which involved simply mixing iron fillings with Sulphur powder, we had to heat the mixture to obtain Iron (II) sulphide compound.
- In making a mixture, all we had to do is add variable amounts of each component. In forming the compound, however, the constituent elements combined based on a fixed ratio by mass. For instance, in the formation of Iron (II) sulphide, iron and Sulphur are known to combine in the ration of 3.5:2. This means that if there is an excess of one constituent, it will remain after the reaction. So we can deduce that compounds have fixed compositions.
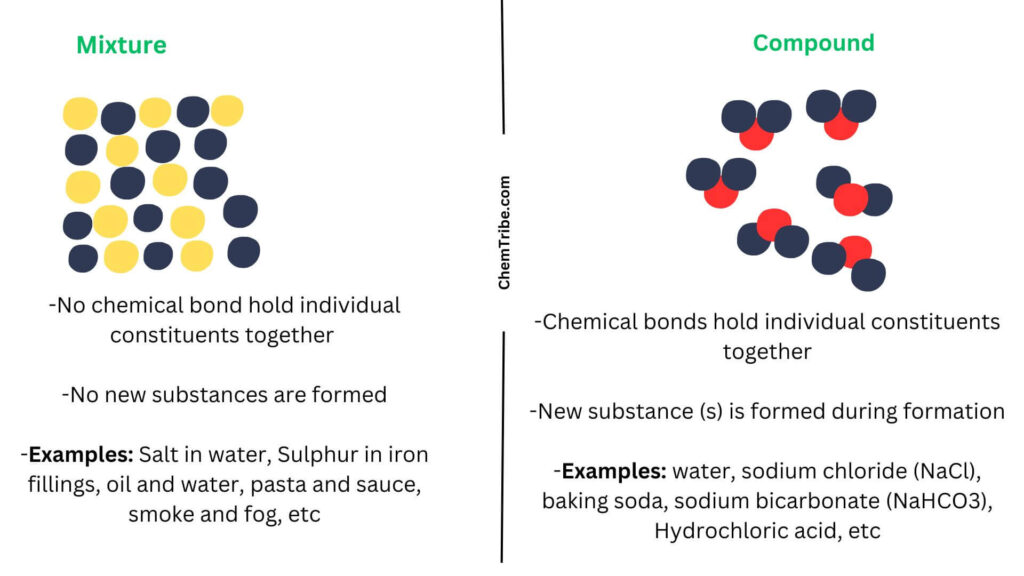
Mixtures Vs Compounds Summary
Here is a summary to help you understand the differences between mixtures and compounds:
| Mixtures | Compounds |
| No new substances are formed during formation | A new substance (s) is formed during formation |
| Can be separated into constituent substances by physical means | Cannot be separated into constituent substances by physical means |
| No chemical changes occurs during formation | Substances combine chemically |
| Components can be in any proportion by mass | Component elements have fixed compositions by mass |
| No heat change occurs during formation | Heat is produced or absorbed during formation |
| Individual components show their unique properties | Properties of new substances are different from those of constituent elements |
| Contains at least two substances | Are single substances |
| Are considered to be impure substances | Are considered to be pure substances |
| No new chemical bond formation takes place | New chemical bonds are formed during formation |
| Cannot be represented by chemical formula | Can be represented by chemical formula (Nacl, KNO3, CaCl2, etc) |
| Examples include Salt in water, Sulphur in iron fillings, oil and water, pasta and sauce, smoke and fog, etc | Examples include water, sodium chloride (NaCl), baking soda, sodium bicarbonate (NaHCO3), Hydrochloric acid, etc |
Further Reading
How to Tell Which Elements Are Present in a Compound from Its Name: The Ate, Ite, & Ide Suffixes
Test Your Knowledge



