Most substances undergo changes when subjected to different conditions such as heat, cold, pressure, electric current, or contact with other substances. These changes can be categorized into two main groups: physical changes and chemical changes. As a chemist, it is essential to know whether a substance undergoes a physical or chemical change for the following reasons:
- Identifying substances accurately in various processes.
- Predicting and controlling reactions for desired outcomes.
- Ensuring laboratory safety by recognizing potential hazards.
- Advancing research and technological innovation through precise characterization of substances and reactions.
Physical Changes
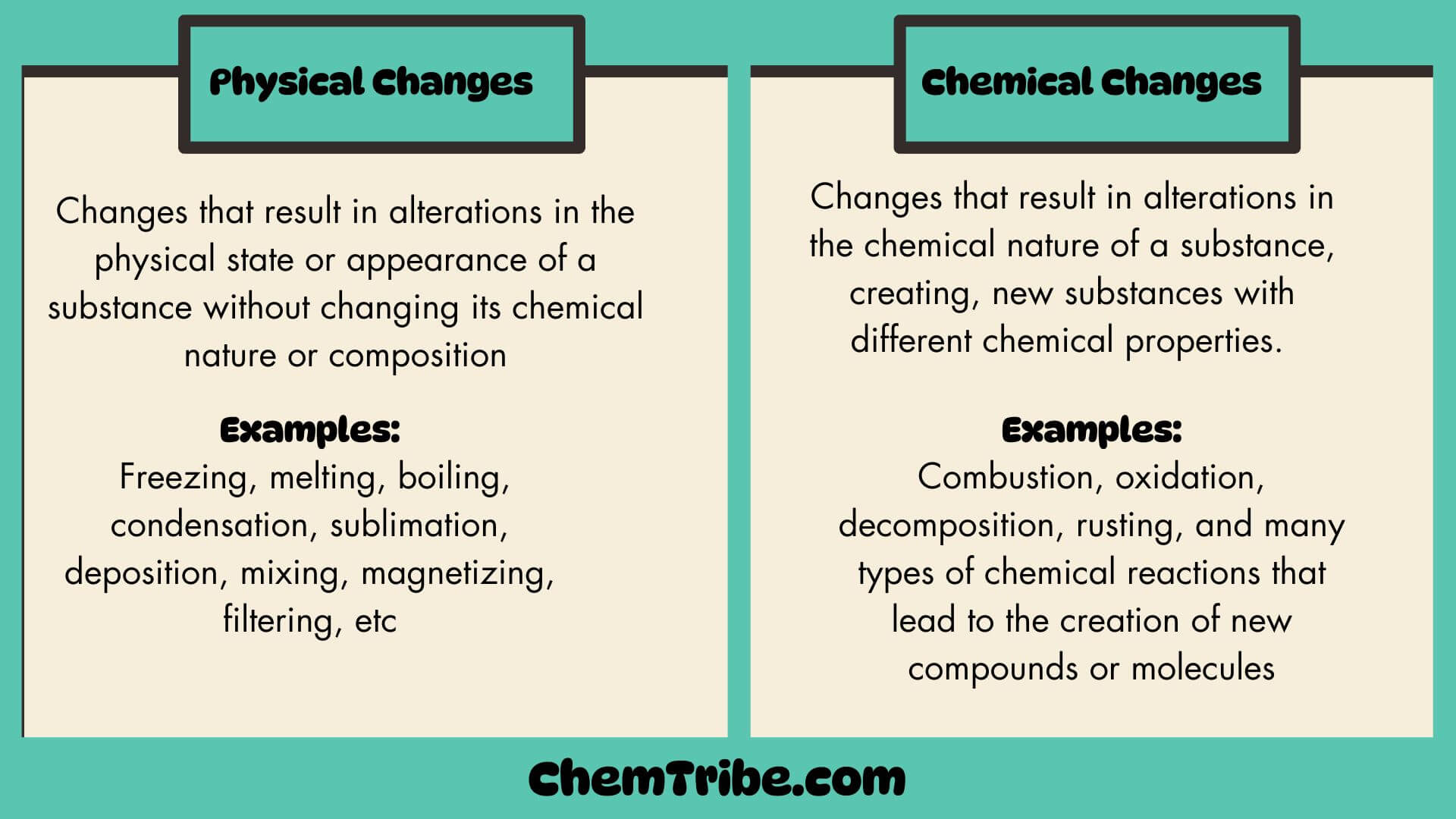
Physical changes are changes that lead to changes in the physical state or appearance of a substance without changing its chemical nature or composition. Since they do not lead to a change in the chemical nature or composition of a substance, no new substances are formed and the changes can easily be reversed. And since they can be easily reversed, they can also be called temporary changes.
Here are some reactions that you can perform in the lab to illustrate physical changes:
I. Boling and cooling of water
Heat some water in a conical flask until it boils and then allow the flaks to cool for some time.
When water is heated it forms steam or vapor. When the vapor is condensed, it forms drops of liquid on the inner walls of the flask.
Liquid Water ⇌ Water Vapor
II. Heating iodine
Place some iodine crystals in a test tube and heat over a Bunsen burner flame.
The black iodine crystals change directly into purple vapor without passing through the liquid state. The vapor changes back to black iodine crystals on the upper cooler parts of the test tube
Solid Iodine ⇌ Iodine Vapor
(Black) (Purple)
III. Heating and cooling of candle wax
Light a candle and place it over a tile. Observe what happens when the molten wax falls on the tile.
The molten candle wax reverts back to its original solid state after cooling.
Solid wax ⇌ liquid wax
IV. Heating solid ammonium chloride
Proceed as II above
The solid ammonium chloride changes directly to while vapor without passing through the liquid phase. The vapor changes back to solid ammonium chloride on the upper, cooler parts of the test tube.
Solid ammonium chloride ⇌ gaseous ammonium chloride
V. Heating Zinc Oxide
Proceed as II above.
Zinc oxide turns to yellow solid on heating and then back to original white solid on cooling.
Solid Zinc Oxide ⇌ Solid Zinc Oxide
(White) (Yellow)
Characteristics of Physical Changes
All the above changes manifest the following general characteristics:
- They are easily reversible (Each substance was easily reversed into its original form on cooling).
- No new substance is formed (The substances changed either their physical states or color but didn’t change chemically)
- The mass of the substances does not change (if we would have weighed the new forms vs the original forms of the substances above, we wouldn’t have observed any mass change in all the above cases—provided that none of the gaseous products were allowed to escape)
- They are not accompanied by net heat change (no marked changes like intense heat or light was observed in all the cases).
Examples of Physical changes
| All changes in state (phase transitions) | Melting, Freezing, Evaporation, Condensation, Sublimation, Deposition |
| Changes in size or shape | Crushing, Cutting, Grinding, Dissolving, Tearing, Bending |
| Changes in appearance | Changing the color or opacity Changing the texture or granularity Changing the state of division (e.g., powdering). |
| Others | Mixing, Magnetizing, Filtering, Sieving |
Chemical Changes
Chemical changes are those that lead to changes in the chemical nature of a substance. Since substances undergo a change in chemical composition, new substances with different chemical properties are formed. The new substances cannot be changed back to the original ones easily.
We have emphasized the term “easily” because it leads us to different types of chemical changes. There are two categories of chemical change: temporary and permanent.
1. Temporary Chemical changes
These are changes that lead to changes in the chemical properties of a substance temporarily, but the new substance can be reversed to its initial state under specific conditions. In other words, these are chemical changes that are reversible.
A good example of a temporary chemical change is heating of hydrated copper (II) sulphate. When hydrated blue crystals of copper (II) sulphate is heated, it decomposes to produce white copper (II) sulphate powder and water.
Hydrated copper (II) sulphate → Anhydrous copper (II) sulphate + Water
(Blue) (White)
When cooled, the white anhydrous copper (II) sulphate does not regain the original blue color.
Anhydrous copper (II) sulphate → Anhydrous copper (II) sulphate
(White) (White)
However, when water is added to anhydrous copper (II) sulphate, it turns to blue. Anhydrous copper (II) sulphate reacts with water to form hydrated copper (II) sulphate, which is a reversal of the above reaction.
Anhydrous copper (II) sulphate + water → Hydrated copper (II) sulphate
(Blue) (White)
We can say that the decomposition of hydrated copper (II) sulphate is reversible and we can represent it as follows:
Hydrated copper (II) sulphate ⇌ Anhydrous copper (II) sulphate + Water
(Blue) (White)
This is a temporary change because the new substances formed (anhydrous copper (II) sulphate and water) are easily reversible to the original substance (hydrated copper (II) sulphate). However, the new substances formed are chemically different from the original substance. That’s why we classify it as a chemical change.
Cobalt (II) chloride also behaves like copper (II) sulphate on heating. Its hydrated form consist of pink crystals, which when heated produces a dark blue solid (anhydrous cobalt (II) chloride and water. Blue anhydrous cobalt (II) chloride turns to pink when water is added.
Hydrated Cobalt (II) chloride ⇌ Anhydrous Cobalt (II) chloride + Water
(Pink) (Blue)
Characteristics of Temporary Chemical Changes
The above two changes manifest the following general characteristics:
- A new substance is formed
- Heat energy is evolved or absorbed
- There is a change in mass
- The change can be reversed
Other examples of temporary chemical changes include acid-base reactions, precipitation reactions, and some types of chemical equilibrium processes.
2. Permanent Chemical Changes
These are changes that lead to changes in the chemical properties of a substance permanently, resulting in the formation of new substances with different chemical compositions. Unlike temporary chemical changes, permanent chemical changes cannot be reversed by physical means or under normal conditions.
A good example of a permanent chemical change is heating of copper (II) nitrate and potassium manganate (VII).
When copper (II) nitrate is heated, it decomposes to form a black (copper (II) oxide) and a mixture of gases (nitrogen (IV) oxide) and oxygen.
Copper (II) nitrate → Copper (II) oxide + Nitrogen (IV) oxide +Oxygen
(Blue) (Black) (Red-brown) (Colorless)
If the mass of copper (II) oxide is measured, it will be lower than that of copper (II) nitrate due to the loss of the gaseous mixture escaping into the atmosphere.
When potassium manganate (VII) is heated, it decomposes into black-green solid and a colorless gas (oxygen). The black-green solid is a mixture of black manganese (IV) oxide and green potassium manganate (VI). If the black-green solid is measured, it will weigh less than the original potassium manganate (VII).
Potassium Manganate (VII) → Potassium Manganate (VI) + Manganese (IV) oxide +Oxygen
(Purple) (Green) (Black) (Colorless)
Characteristics of Permanent Chemical Changes
The above two changes manifest the following general characteristics:
- New substances are formed (the original substance broke into three new substances).
- The change is irreversible (the new substances formed cannot be reversed into the original substance easily).
- The change is accompanied by a change in mass (the new substance formed weighed less than the original because the gases formed were allowed to escape).
- Heat energy is released or absorbed (the original substances had to be heated for the reactions to take place).
Examples of permanent chemical changes include combustion, oxidation, decomposition, rusting, and many types of chemical reactions that lead to the creation of new compounds or molecules.
Physical Vs Chemical Changes Summary
| Physical Changes | Chemical Changes |
| Result in changes in the physical state or appearance of a substance without changing its chemical nature or composition | Result in change in chemical nature or composition of a substance. |
| No new substance is formed (The substances changed either their physical states or color but dodn’t change chemically) | New substances with different chemical properties are formed. |
| Temporary (easily reversible i.e original substance can be recovered) | Permanent (are irreversible i.e. original substance cannot be recovered) |
| The mass of the substances does not change | Are accompanied by a change in mass |
| Are not accompanied by net heat changes | Heat energy is released or absorbed |
| Some examples include freezing, melting, boiling, condensation, sublimation, deposition, mixing, magnetizing, filtering, etc | Some examples include combustion, oxidation, decomposition, rusting, and many types of chemical reactions that lead to the creation of new compounds or molecules |