We have used the terms “physical” and “chemical” properties of substances in previous posts, and we will use them frequently in subsequent posts. Therefore, it is important to understand what they mean in chemistry.
Each substance possesses a unique set of properties that distinguishes it from other substances. These properties can be classified into two main categories:
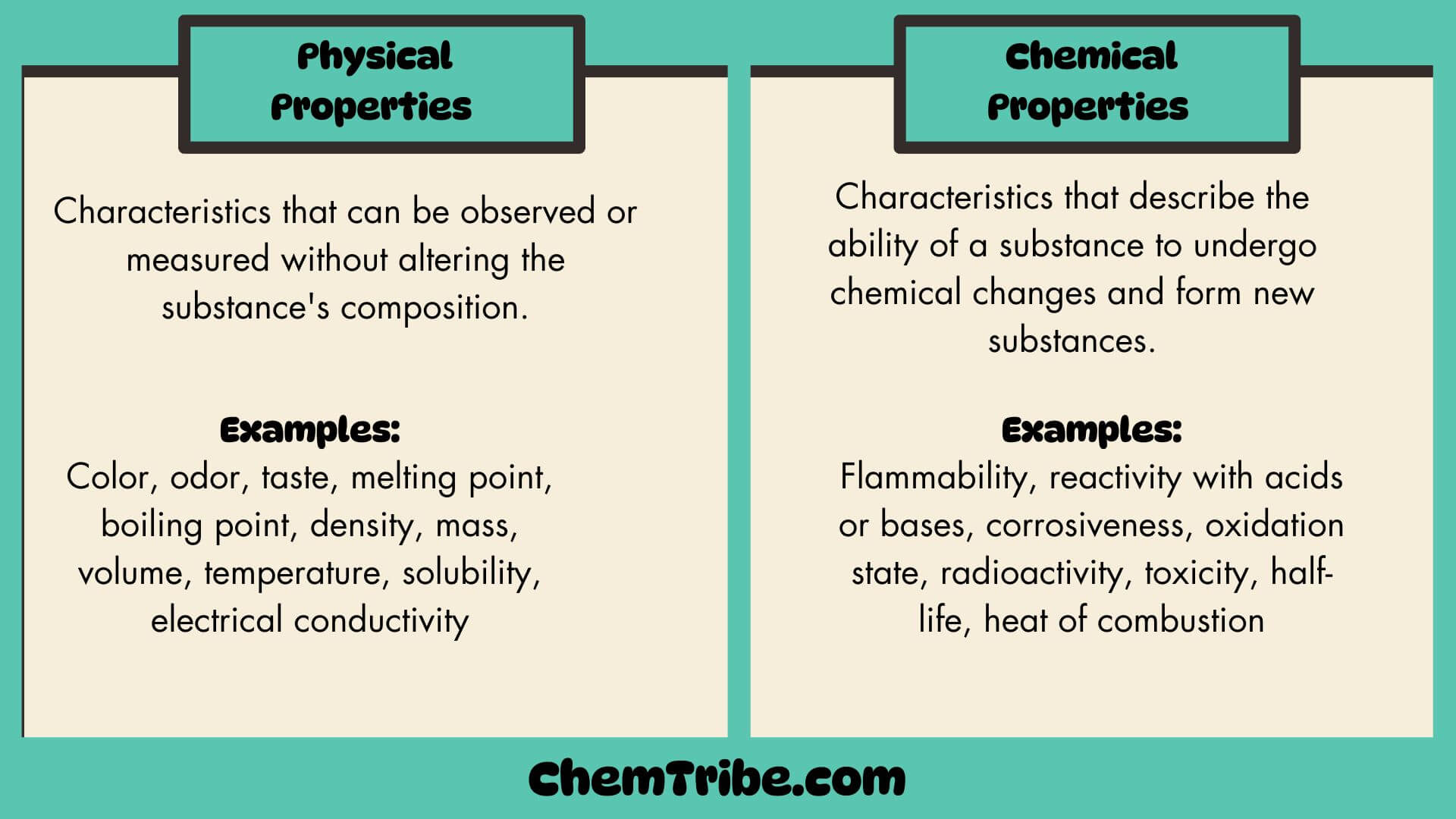
- Physical Properties
- Chemical properties
Physical properties are those properties that a substance shows by itself without interacting with another substance. Examples include things like color, odor, taste, melting point, boiling point, density, mass, volume, temperature, solubility, electrical conductivity, etc.
On the other hand, chemical properties refer to a substance’s ability to interact with other substances or to change into another substance (or substances). For instance, the chemical properties of a metal may be described by its ability to interact with water, oxygen, or acids to form new substance(s). Examples of chemical properties include flammability, reactivity with acids or bases, corrosiveness, oxidation state, radioactivity, toxicity, half-life, heat of combustion, etc
Since each substance possesses unique properties distinct from those of all other substances, it is possible to distinguish it from others. For instance, we know that water is the only liquid that is colorless, odorless, tasteless, with a density of 1g/cm³, freezes at 0°C, boils at 100°C at atmospheric pressure, and reacts rapidly with sodium to produce hydrogen gas.
Here is a summary to help you further understand the differences between physical and chemical properties of matter:
| Property Type | Physical Property | Chemical Property |
| Definition | Characteristics that can be observed or measured without altering the substance’s composition. | Characteristics that describe the ability of a substance to undergo chemical changes and form new substances. |
| Observability | Can be observed or measured directly. | Often require chemical reactions to observe. |
| Examples | Density, color, melting point, boiling point, solubility, conductivity. | Flammability, reactivity with acids or bases, corrosiveness, oxidation state. |
| Change | Physical properties remain unchanged during physical changes (e.g., phase changes). | Chemical properties change during chemical reactions as substances are transformed into new substances with different properties. |
| Measurement | Typically measured quantitatively. | Often assessed qualitatively or through reaction-based tests. |
| Purpose | Used to identify substances and describe their behavior under different conditions. | Used to understand how substances interact with each other and how they transform chemically. |
Related Concept: Physical and Chemical Changes