When working in closed systems where pressure changes can cause problems, the most suitable laboratory equipment for adding controlled amounts of liquid to a reaction flask is not a standard dropping funnel, but a pressure-equalizing dropping funnel (also called a dropping funnel with pressure-equalizing capabilities).
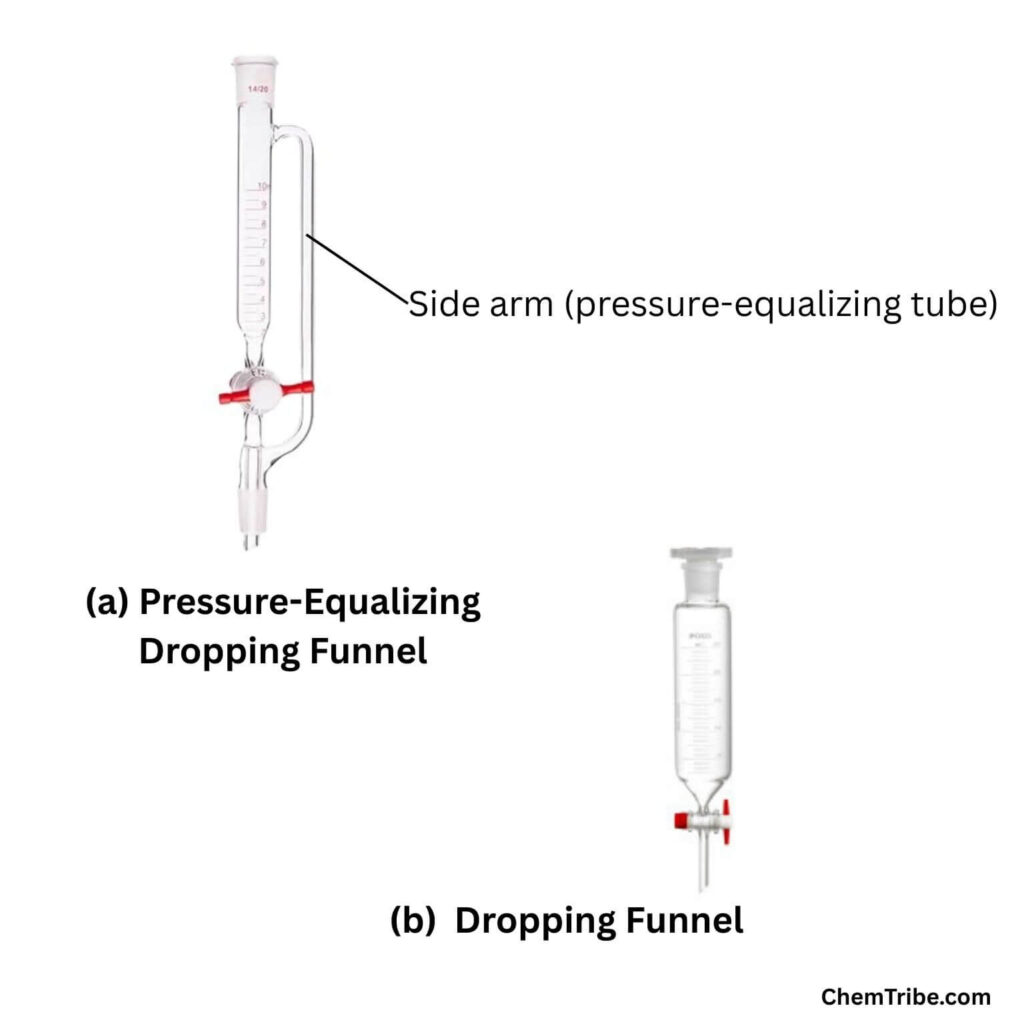
As shown in the diagram above, a pressure-equalizing dropping funnel features a side arm, known as the pressure-equalizing tube, which connects the funnel to the reaction flask. This is the most important feature of the apparatus and is what distinguishes it from a typical dropping funnel.
How Pressure-Equalizing Dropping Funnel Works
When liquid leaves the funnel and enters the flask, the pressure inside the flask naturally drops, which can slow or stop the flow or even cause liquid to flow backward. The pressure-equalizing tube allows air or inert gas from the flask to flow back into the funnel, balancing the pressure and enabling a steady, controlled addition of the reagent.
There are instances when it is important for the pressure in a dropping funnel to remain constant. A perfect example is when adding reactive or volatile chemicals, as maintaining equal pressure helps prevent back suction, which could lead to accidents in the laboratory. Equal pressure also ensures that the liquid being added flows smoothly into the reaction flask. Finally, maintaining consistent pressure is critical when measuring gas volumes accurately, as it reduces the influence of the added liquid on the overall gas pressure in the system.
Uses of Pressure-Equalizing Dropping Funnel
Now that you’ve got a grasp of the basics of the pressure-equalizing dropping funnel, you might be curious about the types of experiments that require these funnels in the laboratory.
As aforementioned in our introduction, pressure-equalizing dropping funnels are essential whenever you need to add liquid reagents to a reaction vessel in a controlled and safe manner, especially in systems that are closed or sensitive to pressure changes.
In experiments where precise, dropwise addition of reactive, volatile, or air-sensitive chemicals is required, a pressure-equalizing funnel ensures that the flow of reagents is steady while preventing vacuum buildup or backflow, making the process safer and more accurate.
Such experiments include but are not limited to:
- Exothermic Reactions: In reactions that release heat rapidly, pressure-equalizing dropping funnels allow reagents to be added slowly and controllably, reducing the risk of runaway reactions or splashing.
- Reactions Under Inert Atmospheres: When working with air- or moisture-sensitive reagents, the funnel enables the addition of liquids without exposing the reaction mixture to the atmosphere, maintaining a protective nitrogen or argon environment.
- Titration or Dropwise Addition Experiments: For reactions that require careful, incremental addition of a reagent, such as in kinetic studies or sensitive titrations, these funnels provide precise control over the flow rate.
- Reflux or Distillation Setups: During reflux or distillation experiments, pressure-equalizing funnels can safely deliver additional reagents to the reaction vessel without disrupting the setup or causing pressure-related accidents.
- Catalysis or Multi-Step Reactions: In reactions involving catalysts or sequential reagent additions, these funnels ensure that reagents are added consistently, helping maintain reaction efficiency and preventing unwanted side reactions.
Safety Guidelines for Using Pressure-Equalizing Dropping Funnel In the Laboratory
When using a pressure-equalizing dropping funnelin the lab, it is crucial to follow a few safety guidelines to prevent accidents:
- Inspect before use: Check the funnel, stopcock, and side arm for cracks, chips, or blockages.
- Secure connections: Ensure a proper fit to the reaction flask using a ground-glass joint; never force the connection.
- Control reagent addition: Open the stopcock slowly to add reagents gradually and prevent overflow or sudden reactions.
- Keep the side arm clear: Make sure the pressure-equalizing tube is unobstructed to allow air or inert gas flow.
- Work under a fume hood: Especially important when handling volatile, toxic, or flammable chemicals.
Apart from these safety tips, strive to adhere to all other laboratory safety rules. Check this post for a quick overview of the basic lab safety rules for students.



