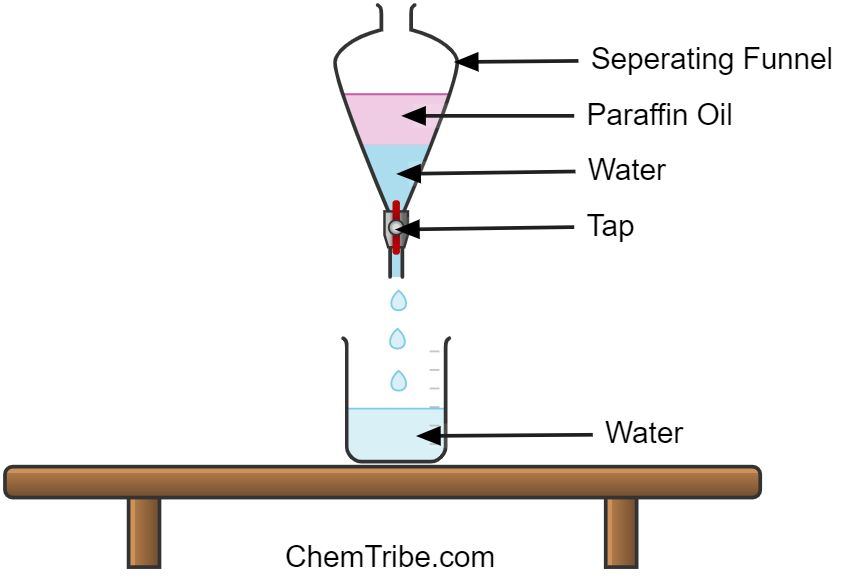
Another separation technique that we can use to separate mixtures is the use of a separating funnel. In particular, a separating funnel can be used to separate a mixture containing two or more immiscible liquids. Immiscible liquids don’t mix. Instead, they form layers with the denser liquid sitting at the bottom of the funnel and the least dense liquid occupying the top. The liquids are then drained off one by one by opening and closing the tap of the separating funnel.
Here is a simple experiment to illustrate this separation technique.
Separating a Mixture of Oil and Water Using a Separating Funnel
Apparatus and Chemicals Needed
- Separating funnel
- Beakers
- Rubber stopper
- Paraffin oil
- Distilled water
Procedure
- Put equal volumes of paraffin oil and water in a separating funnel until it is about half full. Ensure that the tap of the separation funnel is closed.
- Close the mouth of the funnel with a rubber stopper and shake the mixture.
- Allow the mixture to stand until distinct layers or phases are formed.
- Remove the rubber stopper and open the tap to allow the bottom layer to drain into a beaker.
- After most of the bottom layer has drained off, close the tap. Remove the beaker and drain the remaining bottom layers into a separate container and discard it. This helps ensure that there is no part of the top layer that gets into the beaker containing the bottom layer.
- Put another beaker under the funnel and drain the other layer.
Discussion
The first beaker contains only water while the second one has only paraffin oil. So, we have successfully separated them.
Paraffin oil and water are immiscible liquids. After the mixture had settled, the oil and water separated into two layers. The bottom layer contained mainly water because it is denser while the top layer contained oil. The two layers were then separated by draining them off separately.
The interphase contained both water and paraffin oil and it had to be discarded (check the second last step of the above procedure) because it is not easy to separate the two liquids at the interphase.
We could also use decantation to separate this mixture (and any other immiscible liquids) but it is not efficient. First, it requires sufficient settling time for the layers to form, which can prolong the overall separation process. Secondly, there is a risk of re-mixing the layers, especially if the pour is not done carefully or if there is turbulence.
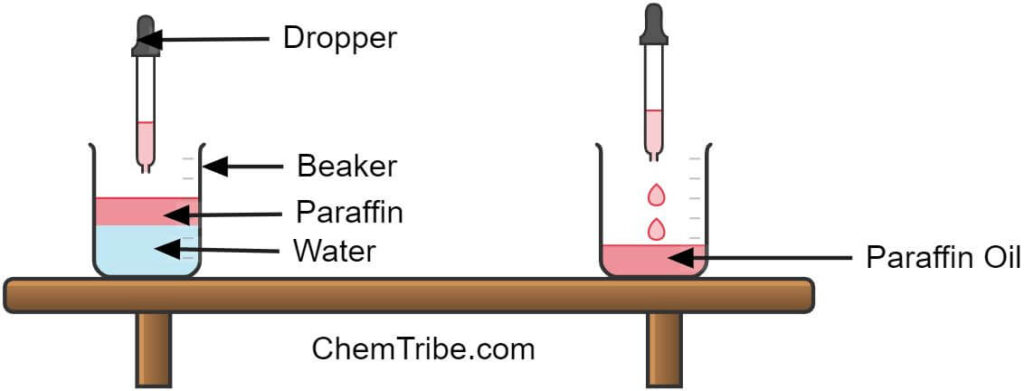
A good alternative to a separating funnel is a dropper. A dropper could also be used to separate these immiscible liquids by sucking the upper layers. The dropper can be used to suck one layer, transferring it into another beaker repeatedly. Using a dropper, too, is less efficient and accurate than using a separating funnel. Using a dropper requires multiple repetitions and careful manipulation to achieve satisfactory separation.



