Solvent extraction is a separation technique that is used to separate components of a mixture based on their solubility in different solvents. It is also referred to as liquid-liquid extraction.
Here is how it works: the mixture is first dissolved in a solvent, and then another immiscible solvent is added. The desired compound selectively dissolves in one of the solvents, while the other components remain in the original solvent. The two immiscible phases are then separated, and the desired compound is recovered from the solvent by other separation techniques that we have covered previously like evaporation, decantation, or filtration.
To help you better understand how a solvent extraction process works, let’s discuss how it can be used to separate a few mixtures, including:
- Separating iodine and sodium chloride mixture
- Extracting caffeine from tea leaves
- Extracting oils from nuts
1. Separating Iodine and Sodium Chloride Mixture
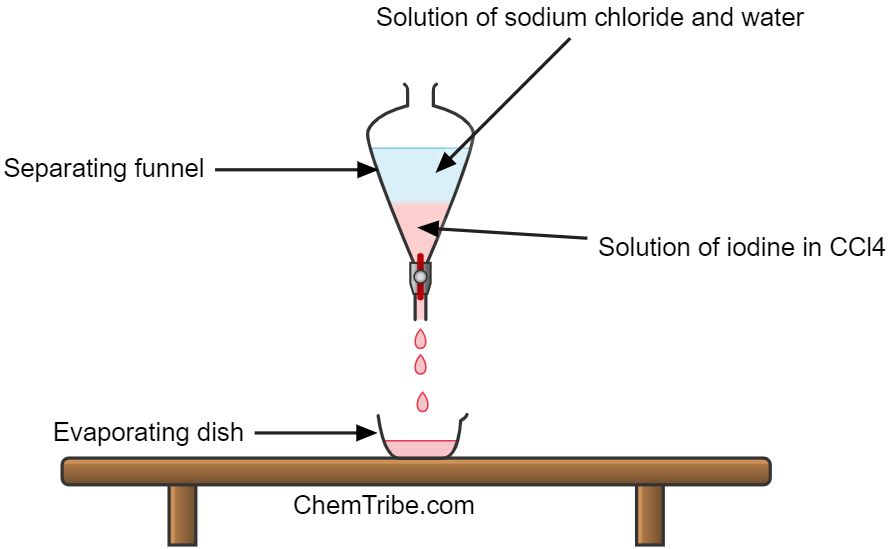
Apparatus and Chemicals Needed
- Beakers
- Evaporating dishes
- Rubber stopper
- Separating funnel
- Iodine
- Sodium chloride
- Carbon tetrachloride (CCl4).
Procedure
- Prepare a solution of iodine and sodium chloride and add it in a separating funnel.
- Add a similar volume of carbon tetrachloride into the separating funnel.
- Close the mouth of the separating funnel with a stopper and shake the mixture
- Leave it to stand for a few minutes until two layers are formed
- Remove the stopper and drain the two layers into separate evaporating dishes
- Heat each dish to evaporate the solvent and obtain the solutes.
Observations and Inferences
Upon evaporation, you will observe that one dish contains a purple powder (iodine) and the other holds a white powder (sodium chloride).
While sodium chloride is highly soluble in water, iodine is only slightly soluble. However, iodine is much more soluble in carbon tetrachloride (CCl4).
Since water and carbon tetrachloride are immiscible, they separate into two layers, with carbon tetrachloride forming the bottom layer due to its higher density.
When the solution of iodine and sodium chloride in water is mixed with carbon tetrachloride, sodium chloride remains in the water while iodine dissolves in carbon tetrachloride.
Consequently, the upper layer consists of a solution of sodium chloride in water, while the bottom layer contains a solution of iodine in carbon tetrachloride.
After separating the two layers, the solvents (water and carbon tetrachloride) are evaporated to recover pure sodium chloride and iodine.
2. Extraction of Caffeine from Tea leaves
Apparatus and Chemicals Needed
- Beakers
- Evaporating dish
- Rubber stopper
- Separating funnel
- Bunsen Burner
- Tripod stand
- Filter paper
- Filter funnel
- Tea leaves chloroform
Procedure
- Boil some tea leaves in water
- Filter the mixture to remain with a filtrate
- Mix some filtrate with equal volumes of chloroform (take care because fumes of chloroform are toxic)
- Add the mixture in a separating funnel
- Close the mouth of the separating funnel with a stopper and shake the mixture
- Leave it to stand for a few minutes until two layers are formed
- Remove the stopper and drain the lower layer into an evaporating dish
- Place the evaporating dish in the open to allow the solvent to evaporate
Observations and Inferences
After some time, you will notice a white solid substance remaining in the evaporating dish. When tea leaves are boiled in water, caffeine and other components (such as flavanols catechins, leucoanthocyanines, phenolic acid, and their derivatives) dissolve in the hot water.
When this solution is mixed with chloroform, caffeine dissolves in the chloroform because it is more soluble in chloroform than in water.
Since chloroform and water are immiscible, they separate into two layers, with chloroform forming the lower layer due to its higher density.
After draining and vaporizing this lower layer, caffeine is left behind as a white solid in the evaporating dish.
3. Extraction of Oils from Nuts and Seeds
Apparatus and Reagents Needed
- Mortar and Pestle
- Evaporating dish
- Propanone
- Groundnuts
Procedure
- Crush some groundnut seeds (or castor oil seeds, sunflower seeds, or cotton seeds) using a mortar and pestle
- Continue crushing the nuts while adding propanone a little at a time
- Decant the resulting solution into an evaporating dish
- Leave the solution in the sun for sometime
- Put a drop of the residual liquid on a piece of paper and hold it against sunlight.
Observations and Inferences
Most of the solution evaporates leaving an oily liquid. The oily liquid leaves a translucent spot on the sheet of paper.
Nuts like groundnuts, cashew nuts, and coconuts contain a significant amount of oil. When they are crushed and mixed with organic solvents like propanone (acetone), the oil dissolves in the acetone. (The nuts are crushed to increase the surface area in contact with the solvent.) This means that the decanted liquid is a solution of oil in propanone.
When this solution is exposed to sunlight, the organic solvent (acetone), which is highly volatile, evaporates, leaving behind the oil.
When a drop of oil is placed on a sheet of paper, it leaves a translucent spot (often used as a simple test for the presence of oils).
The oil obtained this way can be purified by washing the product in water and separating it from the water using a separating funnel.
Practical Applications of Solvent Extraction
Some of the common industrial applications of solvent extraction include:
- Extraction of oils from nuts and seeds.
- Removing unwanted substances from food materials. For instance, it can be used to remove caffeine from coffee to get decaffeinated coffee, which is less addictive.
- Extraction of certain medicine drugs or active ingredients (such as alkaloids and essential oils) from plants (in pharmaceutical industries).
- Removing pollutants, such as heavy metals and organic contaminants, from soil and water during environmental cleanup efforts.
- Recovering valuable materials from industrial waste streams, such as metal ions from spent batteries or electronic waste.
- Preconcentration and separation of trace analytes from complex matrices, such as environmental samples or biological fluids.



