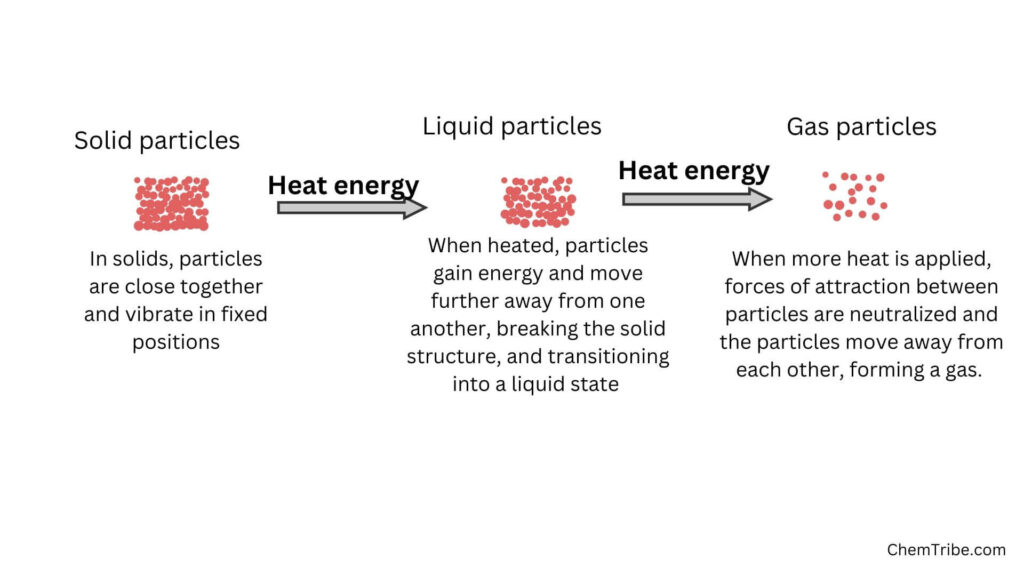
Sublimation is the direct transition of a solid into vapor on heating without passing through liquid state. This occurs when the vapor pressure of the solid exceeds atmospheric pressure at a certain temperature.
The opposite of sublimation i.e the transition from vapor directly to solid, is called Deposition. The solid formed when the vapor cools is called sublimate.
In deposition, the molecules of a gas lose energy and come together to form a solid substance. This occurs when the temperature of the gas decreases below its sublimation point, causing the gas molecules to lose enough energy to form a solid on surfaces.
Sublimation vs Deposition
| Characteristic | Sublimation | Deposition |
| Phase Transition | From solid to vapor directly | From vapor to solid directly |
| Direction | Solid → Vapor | Vapor → Solid |
| Temperature | Occurs at or above the substance’s sublimation temperature | Occurs at or below the substance’s deposition temperature |
| Examples | Dry ice (solid CO2) sublimes at -78.5°C | Water vapor in the air turns into frost on a cold surface |
Only a few substances undergo sublimation. These include:
- Ammonium chloride
- Anhydrous Iron (III) chloride
- Solid carbon (IV) oxide
- Iodine
- Naphthalene (mothballs)
- Benzoic acid
- Camphor
- Arsenic trioxide
- Anthracene
- Solid air fresheners containing fragrant oils
Solids that sublime can be separated from mixtures with other solids that don’t sublime. For example, if a mixture contains a compound or solid that sublimes (sublimable) and another solid or substance that doesn’t sublime (non-sublimable), heating the mixture can cause the sublimable compound to vaporize and leave behind the non-sublimable substance. The vapor can then be collected and cooled to recover the pure sublimable compound in its solid form.
Here is a simple experiment to illustrate how sublimation can be used as a separation technique:
Separating Iodine Crystals from a Mixture of Iodine and Sodium Chloride (Common Salt)
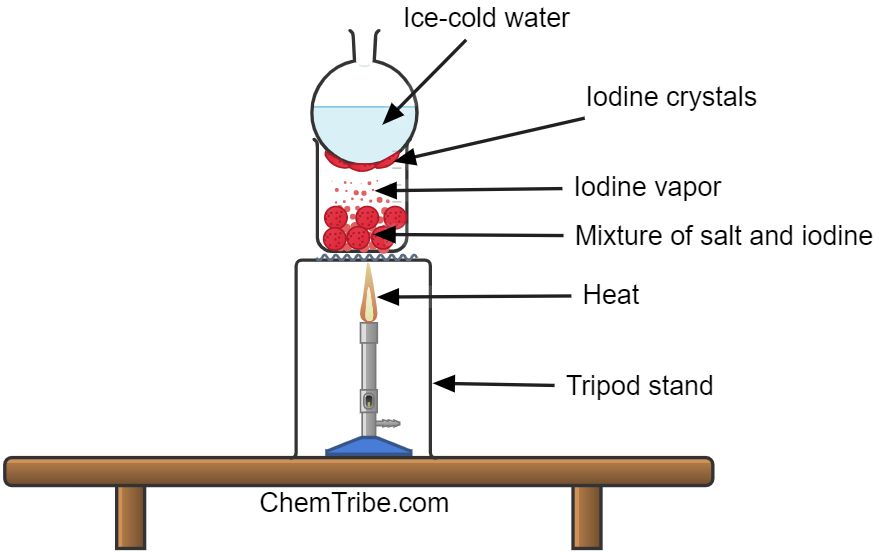
Apparatus and Chemicals Needed
- Tripod Stand
- Pyrex Beaker
- Round-bottomed flask
- Bunsen burner
- Wire gauze
- Sodium chloride
- Iodine
Procedure
- Prepare a mixture of sodium chloride and iodine and add it to a Pyrex beaker.
- Set up the apparatus as shown in the figure above
- Heat the mixture gently and observe what happens
- Precaution: iodine vapor is poisonous and care should be taken not to inhale it.
Observations and Inferences
Dense purple fumes of iodine form in the beaker. The vapor then condenses at the bottom of the round-bottomed flask, producing crystals of iodine. Since sodium chloride does not sublime, it remains in the beaker.
Practical Applications of Sublimation
- Sublimation is used for purification of substances like iodine and camphor by separating them solid mixtures or impurities that don’t sublime.
- Sublimation is used for cooling purposes. For example, solid carbon dioxide sublimes at -78°C. As it undergoes sublimation, it absorbs heat from its surroundings, thus cooling them. This property makes it a popular choice for ice cream and soft drink vendors as a coolant. And since solid carbon dioxide never liquefies, it does not wet the objects it cools, making it easy to handle and transport.
- Sublimation is used in the production of solid air fresheners, where fragrances are encapsulated in a solid matrix and released into the air as they sublime.
- Sublimation is also applied in dye-sublimation printers and copiers to transfer dye onto paper or other materials, resulting into high-quality prints.