Prerequisite Reading: The Three Primary States of Matter in Chemistry
In the previous post, we discussed the three primary or conventional states of matter: solid, liquid, and gaseous states. We mentioned that these are not the only states of matter and promised to reveal the other two most important states of matter in chemistry: the 4th and 5th states of matter, plasma and Bose-Einstein condensate states.
Without further ado, let’s discuss these states of matter.
Plasma
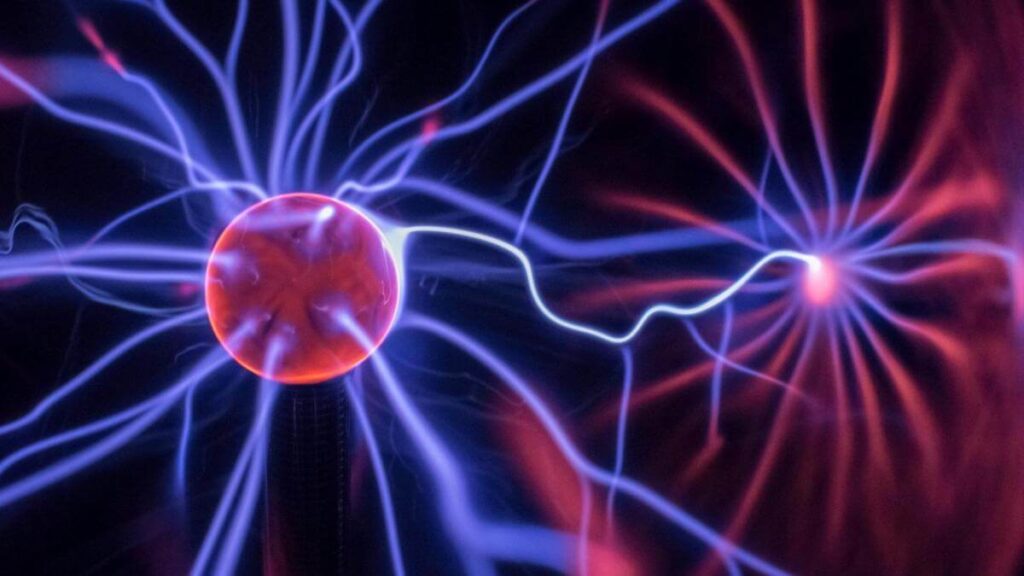
Plasma is sometimes called the fourth state of matter. Like gases, they don’t have definite shapes or volumes.
Just as liquid water can be transformed into steam (a gaseous state) by heating or adding energy, heating a gas will convert it into a super-heated mixture of ions (positively charged particles) and electrons (negatively charged particles). This combination of ions and electrons is what we call the plasma state. Simply put, plasma is matter that is superheated and has high kinetic energy. Matter in this state is considered ionized because electrons have been stripped away from the atoms.
Compared to the solid, liquid, and gaseous states, plasma isn’t very common here on Earth. That’s why you may not observe many things around us in a plasma state. However, it is the most common and abundant state of matter in the visible universe, making up to 99 % of the universe.
Neon signs are perfect examples of things that are in the plasma state. The glowing signs are created when noble gases (helium, neon, argon, krypton, or Xenon, etc.) are ionized using electricity to plasma states. Other examples of things around us that exist in the plasma state include the sun, stars, lightning, electricity, flames, auroras, nebulas, electric sparks, fluorescent lights, plasma televisions, plasma balls, St. Elmo’s fire, etc.
Bose-Einstein Condensate
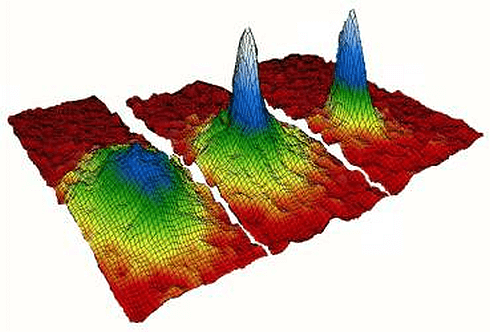
The fifth state of matter, Bose-Einstein condensate, is perhaps the most mysterious. It is created when groups of particles are cooled to near absolute zero (0 kelvins or -273.150). At such low temperatures, the particles can hardly move relative to each other because they almost have no free energy to do so. Consequently, they clump together and enter the same energy states. As a result, the whole group of particles becomes a single super-particle cloud. From a physical point of view, the particles become identical and start to behave as if they were a single atom.
BEC was first predicted in 1924-1925 by Albert Einstein based on the work of Satyendra Nath Bose on quantum statistics hence the name. The experimental creation of BEC occurred in 1995 by Eric Cornell and Carl Wieman using rubidium, followed by Wolfgang Ketterle’s achievement with sodium atoms. This groundbreaking work earned them the Nobel Prize in Physics in 2001.
To create a Bose-Einstein condensate, a cloud of diffuse gas, often atoms like rubidium or strontium, is cooled using lasers and evaporative cooling until the atoms clump together and enter the same energy states, behaving collectively as if they were a single atom. This unique state of matter follows Bose-Einstein statistics, where particles are indistinguishable from each other.
Since we’ve been listing for you some examples of the states of matter we’ve discussed so far, you’re probably wondering: where would you find a Bose-Einstein condensate or what are some examples of Bose-Einstein condensates?
Well, the first place that comes to mind is in the lab. Various laboratories use lasers and evaporative cooling to cool clouds of diffuse gases, typically rubidium or strontium until they reach the BEC state. For example, the Killian Lab created a Bose-Einstein condensate from strontium in 2009, and Aalto University achieved the same in 2018. Additionally, NASA launched a Cold Atom Lab into the microgravity of the International Space Station to produce BECs.
You can also find BECs in ultracold atomic gases, as well as in quasiparticles or integer spin particles like magnons, excitons, and polaritons, especially at high temperatures. These quasiparticles behave as bosons, allowing them to form condensates.
Other Exotic States of Matter
Matter also exists in other exotic states beyond these five, such as the symmetric crystalline state, superconductors, superfluids, fermionic condensates, Rydberg molecules, photonic matter, and dropletons, among others. However, these are beyond the scope of this post, so we won’t delve into them.
Test Your Knowledge
- Changes of States of Matter Worksheet
- States of Matter Quiz
- States of Matter Crossword Puzzle
- States of Matter Word Search
Image Credits: 1, 2



