Prerequisite Reading: The Three Primary States of Matter in Chemistry
The kinetic theory of matter is a scientific concept that is often used to understand the behavior of matter, especially with respect to the motion of its constituent particles.
According to the theory, matter is composed of particles that are in a continuous state of motion. Due to this motion, the particles possess kinetic energy. Additionally, the particles attract one another, which tends to keep them together.
When a substance transitions from one state to another, the particles do not change chemically, but their speed of motion and the force of attraction between them change, along with their arrangement.
The kinetic theory forms the basis of the theoretic model of matter. As such, we can use it to explain the three states of matter we discussed previously as well as their transitions from one state to another.
Explanation of the Behaviors of Particles in the Three States According To Kinetic Theory
Solid State
In the solid state, the particles are close to each other and are held together by strong attractive forces. The particles can’t move freely but can only vibrate in fixed positions. As a result, particles in solids are packed in a uniform pattern characteristic of each substance. This accounts for the fixed shape and volume of solids, which can only be changed by the application of a strong force.
Liquid State
In the liquid state, the particles are positioned further apart as compared to the solid state. They are free to move about but cannot break away because they are still attracted to one another. This free movement of particles in liquid state accounts for the ability of liquids to flow and conform to the shape of the container. But since the particles cannot break away, liquids maintain fixed volumes.
Gaseous State
Finally, in the gaseous state, the particles are positioned very far apart from one another and move independently in all directions at great speeds. Unlike the liquid and solid states, the attraction between the particles is negligible. Because of the random and fast motion of particles, gases spread out fast and fill the container in which they are placed.
Explanation of the Changes of State
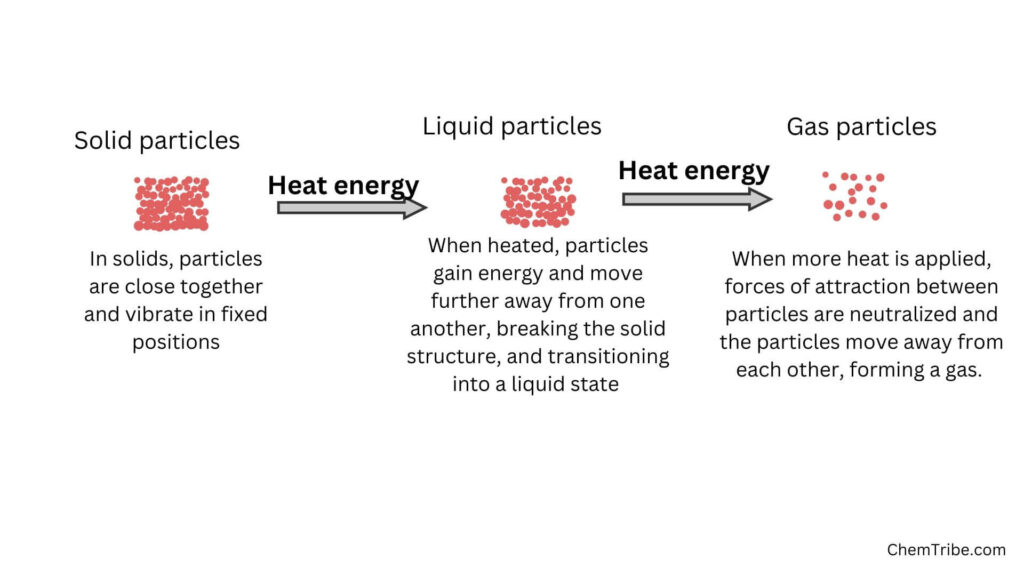
Whether a substance exists in a solid, liquid, or gaseous state depends on the amount of kinetic energy that its particles possess, which tends to disperse them, and the force of attraction between the particles, which tends to bring them together.
Heating
When solids are heated, their constituent particles gain kinetic energy, making them to vibrate more vigorously. When heating is continued, the particles gain more energy to overcome the force of attraction between them. This causes them to break away from the solid structure. They start to melt and transition to the liquid state.
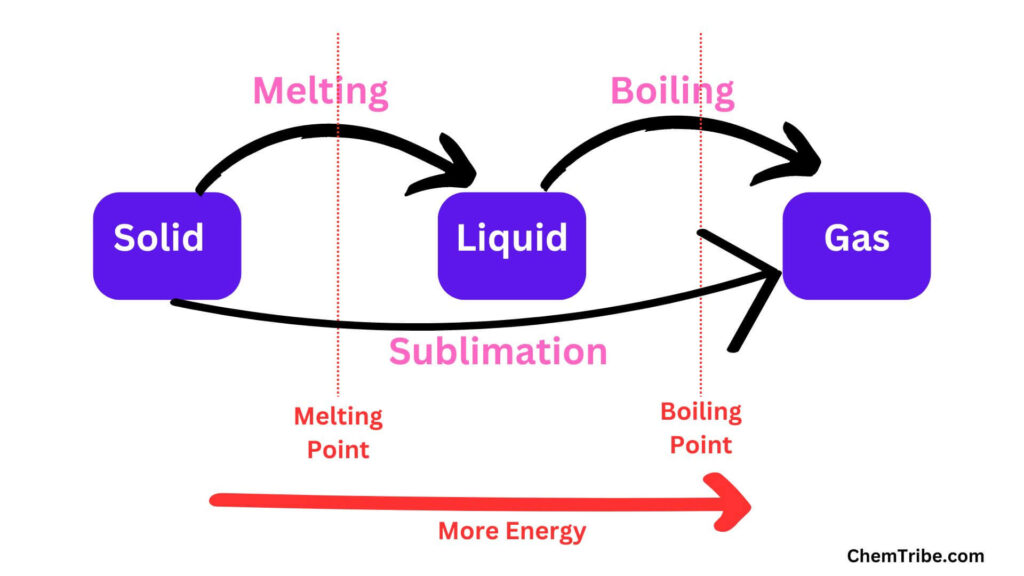
Melting occurs at a temperature called the melting Point. At the melting point, the particles possess enough energy to overcome the forces that are holding them together. As a result, they begin to move around and vibrate more.
When heating is continued, the particles in the liquid gain more kinetic energy and move faster. A point is reached when the kinetic energy of the particles increases so much that it completely neutralizes the force of attraction between the liquid molecules. The particles break away from one another and begin to escape. This point or temperature is called the boiling Point. The liquid starts to boil and gradually transitions to the gaseous state.
We also have substances that transition straight from solids to gases when heated—without passing through the liquid state. This is called sublimation. Examples of such solids include iodine, ammonium chloride, and solid carbon (IV) oxide (or dry ice).
In such solids, the force of attraction between their particles is considerably weak. So, as soon their particles absorb heat energy, they gain a sufficiently high kinetic energy, which causes them to break away from their slid structures into gaseous states.
Cooling
The reverse happens when gasses are cooled. As the temperature falls, the particles lose kinetic energy and move slowly. As the kinetic energy decreases, the force of attraction between the particles increases, making them to come close to one another. A point is reached when they come very close and the gas transitions to a liquid state. The liquid is said to condense and the process is called condensation.

The temperature at which condensation occurs is usually the boiling point of the substance. Sounds confusing? Well, the temperature at which a liquid transitions into a gas is the same temperature at which the gas transitions back to liquid when cooled.
If temperature is allowed to fall further, the kinetic energy of the particles becomes less and they move more slowly. Their forces of attraction become stronger. A point is reached when the particles come together. At this point, they are no longer free to move but vibrate in fixed positions. The liquid transitions to a solid state. This process is referred to as freezing and the point or temperature when this happens (i.e the particles are no longer free to move but vibrate in fixed positions) is the melting point (see explanation in the previous paragraph).
Substances that sublime (iodine, ammonium chloride, dry ice, etc) can also transition from gas to solid states on cooling. This also called sublimation.
Evaporation vs Boiling
Both evaporation and boiling involve a change of state in a substance from liquid to gas. For instance, when water boils, it changes to steam (which is a gaseous state). When water evaporates, it also turns into water vapor (which is also a gaseous state).

So, what is the difference?
Well, while boiling takes place at a specific temperature (for instance, 1000C, for water), evaporation takes place even at lower temperatures—sometimes far below the boiling point.
You’ve probably observed pools of water over tarmac roads evaporating on sunny days even if the air temperature is far below 1000C.
How can we explain this using the kinetic theory of matter?
Well, evaporation occurs because all the particles in a liquid don’t have the same kinetic at a given temperature. Some have higher and others have lower kinetic energies. When particles with higher kinetic energies come to the surface of the liquid, they break away from the rest of the particles and escape or transition into the gaseous state.
So, evaporation takes place at all temperatures. But its rate can be increased by adding more energy or increasing the temperature.
Recap of Change of State Terminologies
| Term | Definition |
| Melting | The process in which a solid substance changes into a liquid state due to increased temperature. |
| Melting Point | The specific temperature at which a substance changes from solid to liquid. |
| Boiling | The transformation of a liquid into a vapor state, typically occurring when the liquid reaches its boiling point. |
| Boiling Point | The temperature at which a liquid changes into a vapor at atmospheric pressure. |
| Evaporation | The process by which a liquid changes into a gas at temperatures below its boiling point. |
| Sublimation | The direct transition of a substance from a solid state to a gas state (and vice versa) without passing through the liquid phase. |
| Condensation | The conversion of a vapor or gas to a liquid state. |
| Freezing | The process in which a liquid changes into a solid state due to decreased temperature. |
Further Reading:
Learn How to Identify States of Matter with These Examples
How to Demonstrate That Gases Have Mass