The term atomic radius generally refers to the distance from the center of an atom’s nucleus to the outermost shell of its electrons. However, determining the exact size of an atom is challenging because atoms do not have sharply defined boundaries. Moreover, it’s impossible to isolate a single atom and measure its radius directly.
Therefore, the atomic radius can only be described as an effective size—essentially the distance of closest approach between two atoms, depending on the type of bonding involved. For this reason, chemists define various types of radii, such as covalent, van der Waals, ionic, and metallic radii, each corresponding to a specific bonding context. In this post, we will focus on metallic radii.
Metallic Radii
A large proportion of metals are tightly packed and held together by metallic bonds. This dense arrangement of metal atoms forms what is known as a crystal lattice or metallic lattice. The degree of packing, however, varies from one metal to another.
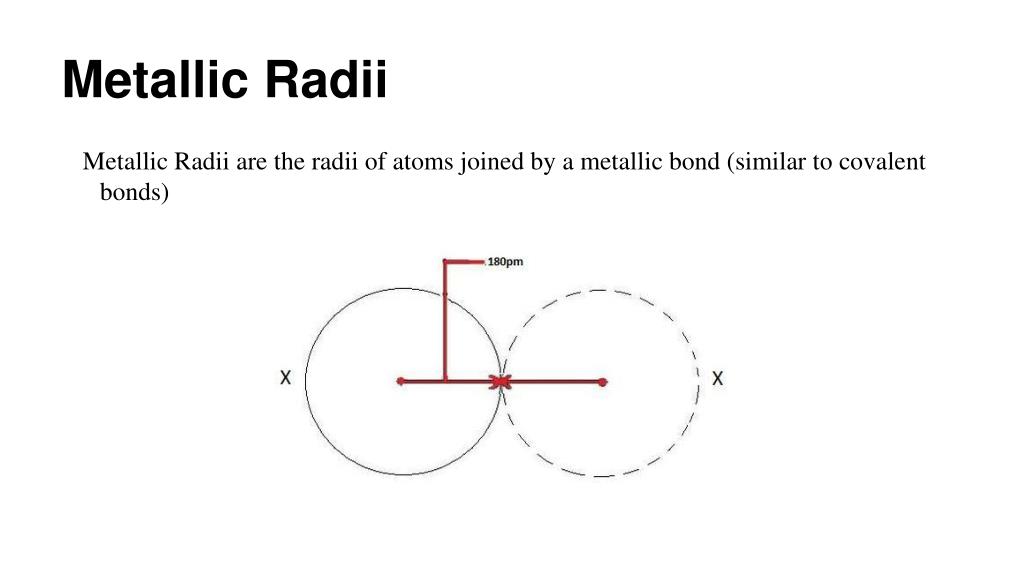
The metallic radius is defined as half the distance between the nuclei of two adjacent atoms within a metallic lattice.
If you’re wondering how this is determined, the distance between the nuclei of nearest neighboring atoms in a metallic crystal is usually measured using X-ray diffraction. Dividing this internuclear distance by two gives the metallic radius.
In general, metallic radii are larger than the corresponding single-bond covalent radii. For example, sodium has a covalent radius of 154 pm, whereas its metallic radius is 186 pm. This difference arises because metallic bonds are typically weaker than covalent bonds, so metal atoms in a metallic lattice are not packed as closely together as they are in covalent structures.



