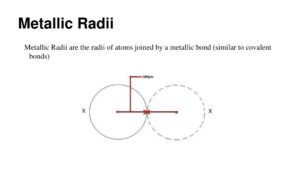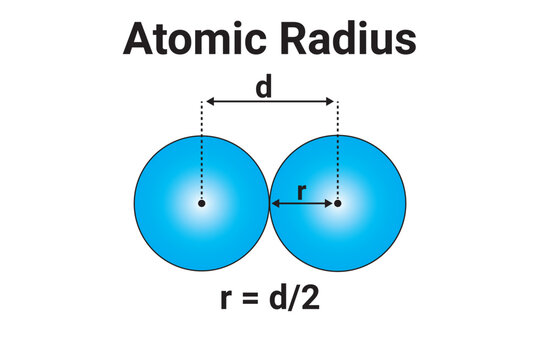
In the previous section, we saw that since the exact size of an atom is difficult to determine, chemists use different types of atomic radii—such as covalent, van der Waals, ionic, and metallic radii—each defined according to the type of bonding involved.
There is also the Bragg–Slater radius, which provides another way to estimate atomic sizes. Let’s take a brief look at it…
Bragg and Slater proposed a set of internally consistent atomic radii. According to their approach, these radii can be added—regardless of the type of bond between two atoms—to closely match the observed internuclear distances.
In essence, Bragg–Slater radii represent the sizes of the inner electron cores, which largely determine how close two atoms can approach each other.
Below are the Bragg–Slater radii for the elements in the second period:
| Element | Covalent Radius (pm) | Bragg–Slater Radius (pm) |
|---|---|---|
| Li | 123 | 145 |
| Be | 89 | 105 |
| B | 80 | 85 |
| O | 66 | 60 |
| F | 64 | 50 |